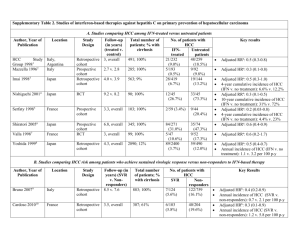
Hsu et al., 1 Postoperative peg-interferon plus ribavirin associated
advertisement

Hsu et al., 1 Postoperative peg-interferon plus ribavirin associated with reduced recurrence of hepatitis C virus-related hepatocellular carcinoma Short title: Postoperative Peg-IFN plus RBV and recurrent HCC Yao-Chun Hsu, MSc, MD1,2, Hsiu J. Ho, PhD3, Ming-Shiang Wu, MD, PhD 4, Jaw-Town Lin, MD, PhD 2,3,5, Chun-Ying Wu, MD, PhD, MPH, LL.M1,6-9 1 Graduate Institute of Clinical Medicine, China Medical University, Taichung; 2 Department of Internal Medicine, E-Da Hospital/I-Shou University, Kaohsiung; 3 School of Medicine, Fu Jen Catholic University, New Taipei; 4 Department of Internal Medicine, National Taiwan University Hospital, Taipei; 5 Center for Health Policy Research and Development, National Health Research Institutes; Miaoli; 6 Division of Gastroenterology, Taichung Veterans General Hospital, Taichung; 7 Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei; 8 College of Public Health, China Medical University, Taichung; 9 Department of Life Sciences, National Chung-Hsing University, Taichung; all in Taiwan * Correspondence: Hsu et al., 2 Chun-Ying Wu, MD, MPH, PhD Faculty of Medicine, School of Medicine, National Yang-Ming University No. 155, Section 2, Linong Street, Taipei 11221, Taiwan E-mail: chun@vghtc.gov.tw; Tel: +886-4-23592525 # 3304; Fax: +886-4-23741331 Jaw-Town Lin, MD, PhD, Chair Professor School of Medicine, Fu Jen Catholic University No. 510, Zhongzheng Rd., Xinzhuang Dist., New Taipei City, 24205, Taiwan E-mail: jawtown@gmail.com; Tel: +886-2-23562246; Fax: +886-2-23947899 Wu CY and Lin JT contributed to this work as corresponding authors. Financial support: This work was supported in part by Taiwan National Health Research Institutes (PH-100-PP-54, PH-101-PP-23). Word Counts: 266 in the abstract; 4664 in the main text (including references) Preliminary results of this study was presented at the 63rd annual meeting of the American Association for the Study of Liver Diseases (the Liver Meeting ® 2012) on November 12, 2012; Boston, MA. Hsu et al., 3 ABSTRACT Hepatocellular carcinoma (HCC) frequently recurs after surgical resection. This population-based research aimed to investigate the association between postoperative antiviral treatment and risk of recurrent HCC in patients with hepatitis C virus (HCV) infection. By analyzing the Taiwan National Health Insurance Research Database, we initially screened a total of 100,938 patients diagnosed with HCC for the first time between October 2003 and December 2010. Among 2,237 antiviral-naïve HCV-infected patients who curatively resected HCC, there were 213 patients receiving antiviral treatment with pegylated interferon plus ribavirin for 16 weeks or more after surgery (treated cohort). These treated patients were matched 1:4 with 852 controls who never treated HCV infection (untreated cohort), in age, gender, cirrhosis, and the elapsed time between surgery and antiviral therapy. Cumulative incidences of and hazard ratios for recurrent HCC were calculated after adjusting for competing mortality. The recurrence rate of HCC was significantly lower in the treated than untreated cohort, with 52.1% (95% confidence interval [CI], 42.0-62.2%) and 63.9% (95% CI, 58.9-68.8%) after 5 years of follow-up, respectively (p=0.001). The number needed to treat for one fewer recurrent HCC at 5 years was 8. The association between postoperative antiviral treatment and risk of recurrent HCC was independent to adjustment for multiple covariates, with an adjusted hazard ratio of 0.64 (95% CI, Hsu et al., 4 0.50-0.83). Stratified analyses revealed that the attenuation in recurrence risk was greater in patients younger than 60 years and those without cirrhosis or diabetes. Conclusion: Postoperative pegylated interferon plus ribavirin is associated with reduced recurrence of HCC in patients with HCV infection. Age, liver cirrhosis, and diabetes mellitus appear to modify this association. Keywords: hepatocellular carcinoma, chronic viral hepatitis C, antiviral therapy Hsu et al., 5 Hepatocellular carcinoma (HCC) is the 3rd most lethal cancer worldwide, causing approximately 600,000 deaths every year. The incidence is highest in Eastern Asia and sub-Saharan Africa, but appears to rise in North America (1, 2). Almost all HCCs occur in the background of chronic liver diseases that include viral hepatitis, alcoholic liver disease, and steatohepatitis (3). Chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) accounts for most HCCs; chronic hepatitis C (CHC) is the leading etiology in countries where prevalence of HBV infection is low (4, 5). Surgical resection is potentially curative for HCC and has been recommended as the treatment of choice if the hepatic reserve permits complete resection (6, 7). Nevertheless, recurrence is very common and strikes 50~60% of patients 3 years after operation (8, 9). In addition to insidious intra-hepatic spreading prior to surgery, a large proportion of recurrent HCCs originate from de novo tumor clones distinct to the resected ones (10, 11). This may result from the underlying liver disease that continuously promotes hepatocellular carcinogenesis despite removal of the primary tumor. There remains a huge unmet need for effective therapy to prevent postoperative recurrence (6, 7). Antiviral therapy may reduce the risk of HCC in patients with chronic viral hepatitis through elimination of viral oncoprotein, resolution of hepatic inflammation, and amelioration of the carcinogenic microenvironment (12-14). A growing body of Hsu et al., 6 evidence has indicated that interferon-based antiviral regimen may decrease development of HCC in CHC patients, particularly in those achieving sustained virological response (15, 16). Nonetheless, viral clearance cannot prevent all HCCs, especially in those with old age or severe liver fibrosis (17), implicating that antiviral therapy may be too late to halt hepatocarcinogenesis in patients with advanced disease. The recurrence rate after HCC resection remained unknown in CHC patients receiving postoperative pegylated interferon (peginterferon) plus ribavirin, the standard anti-HCV regimen for a decade (18). Moreover, it has not been clarified whether this antiviral regimen administered postoperatively was associated with fewer HCC recurrences. Therefore, we aimed in this population-based study to determine the recurrence rate of surgically resected HCC after postoperative administration of peginterferon plus ribavirin, and to elucidate whether this antiviral therapy was associated with reduced recurrence of HCC in CHC patients. PATIENTS AND METHODS Study design and patient population This open-cohort research utilizes population-based data from the Taiwan National Health Insurance Research Database (NHIRD). Since the National Health Insurance is a compulsory universal program for all residents in Taiwan, NHIRD is a comprehensive Hsu et al., 7 healthcare database that nearly covers the entire 23.7 million population of this country. Details regarding the NHIRD have been reported in our previous investigations (19-21). The present study was approved by the Research Ethics Committee of the National Health Research Institutes, Taiwan (EC1010303-E). We firstly screened all patients who had a first-time diagnosis of HCC from October 1, 2003 to December 31, 2010, and then identified the study population as those with CHC who underwent curative surgery. This research defined disease status principally on the basis of admission diagnoses, which were coded according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Apart from the specific ICD-9-CM code (155.0), the diagnosis of HCC had to be certified in the Registry for Catastrophic Illness Patient Database (RCIPD), a subpart of NHIRD. Given that all enrolled patients resected their HCC, histopathological confirmation was required for registry in the RCIPD. All enrolled patients received liver resection as the sole HCC treatment. Those who underwent liver transplantation, local ablation (ethanol injection, radiofrequency ablation, or microwave coagulation), or trans-arterial chemoembolization before or during the index admission were excluded. Patients with metastasis or any other malignant disease were excluded. We enrolled exclusively patients coded with CHC at admission (ICD-9-CM codes: 070.41, Hsu et al., 8 070.44, 070.51, 070.54, V02.62) to ascertain validity of the diagnosis. Those with HBV infection or other viral hepatitis were excluded (ICD-9-CM codes: 070.2, 070.3, V02.61, V02.69). Antiviral therapy and definition of study cohorts The antiviral regimen consisted of peginterferon alpha (either 2a or 2b) plus ribavirin, which has been reimbursed for HCV infection in Taiwan since October 1, 2003. Generally, treatment was initiated at 180 µg per week irrespective of body weight for peg-interferon alpha 2a, 1.5µg/kg per week for 2b, and 800 to 1,200 mg per day for ribavirin, but it was individualized at the treating physician’s discretion and frequently adjusted along the course. The reimbursed duration ranged from 16 weeks to 48 weeks, depending on the date of administration, viral genotype, serum viral load, on-treatment virological response, and patient tolerability (18). The treated cohort comprised antiviral-naïve patients who received peginterferon and ribavirin for a minimum of 16 weeks after surgery. Each treated patient was matched in age, gender, and cirrhosis with 4 untreated counterparts randomly selected from those who never used interferon or ribavirin. Furthermore, the untreated controls were deliberately matched for the time period between surgery and administration of antiviral therapy in treated patients, in order to eliminate the immortal time bias (22, 23). Hsu et al., 9 Postoperative follow-up and definition of HCC recurrence The treated and untreated cohorts were followed up after initiation of antiviral regimen and matched postoperative duration, respectively, until recurrence of HCC, death, or December 31, 2010; whichever occurred first. Recurrence of HCC was defined as repeated cancer treatment for HCC during the follow-up period. Treatment modalities for HCC recurrence included liver transplantation, surgical resection, focal ablation, trans-arterial chemoembolization, radiotherapy, and chemotherapy. HCC that recurred within 3 months of the index surgery was not included because it might arise from incomplete primary resection. Assessment of and adjustment for confounding factors All comorbidities listed in the Charlson’s index were considered as important covariates that might confound outcomes (24). The age-unadjusted Charlson scores were computed for both the treated and untreated cohorts. Certain medications including statin, non-steroidal anti-inflammatory drug (NSAID), aspirin, and metformin were also assessed as potential confounders because they might modify the risk of cancer (19-21). Users of these drugs were defined as those who took them on a regular basis with frequency of more than one tablet per month during the study period. The extent of hepatic surgery, namely major (at least 3 segments of hepatic parenchyma) or minor resection (2 or fewer segments of liver), was also analyzed. Hsu et al., 10 Data analysis and statistical test The primary and secondary outcomes were HCC recurrence and mortality, respectively. Death occurring prior to HCC recurrence, which could lead to informative censoring, was regarded as a competing risk event in estimating the incidence of recurrent HCC. The number needed to treat (NNT) represented the number of patients needed to be treated in association with one fewer recurrent HCC or death. NNT was calculated by the inverse of the absolute risk reduction. The modified Kaplan-Meier method and the Gray's method were used to calculate and to compare the cumulative incidences in data with competing risks (25). After confirming the assumption of proportional hazards by plotting the graph of the survival function versus the survival time and the graph of the log (-log(survival)) versus the log of survival time, we applied the modified multivariate-adjusted Cox proportional hazard model in the presence of competing risks to examine the independent risk factors for HCC recurrence (26). The influence of antiviral therapy on HCC recurrence was further explored in stratified analyses according to age, gender, cirrhosis, comorbidity, medications, and extent of resection. All data was managed with the SAS software 9.2 version (SAS Institute., Cary, NC, USA). The cumulative incidence and hazard ratio (HR) in the competing risk analysis were calculated by using the R software with the “cmprsk_2.1-4” package (by Gray; http://biowww.dfci.harvard.edu/~gray/). Calculated Hsu et al., 11 results were expressed with the estimated numbers alongside their 95% confidence intervals (CIs). All statistical tests were two-sided with significance set at a p value <0.05. RESULTS Baseline characteristics of the study population We screened a total of 100,938 patients diagnosed with HCC for the first time during the study period and finally identified 2,237 CHC patients who underwent curative resection for HCC (Figure 1). Among the 239 patients who ever received peginterferon plus ribavirin after surgery, 213 patients (89.1%) were treated for a minimum of 16 weeks and formed the treated cohort, whose mean (± standard deviation) duration of antiviral regimen was 25.99 ± 8.13 weeks and that of follow-up was 2.01 ± 1.67 years. The matched controls accordingly comprised 852 untreated patients randomly selected from those not receiving antiviral therapy. The untreated cohort was followed up for 1.51 ± 1.28 years. These two cohorts were generally comparable in baseline characteristics (Table 1). HCC recurrence and mortality between the study cohorts HCC recurred cumulatively in 16.2% (95% CI, 10.9-21.4%), 41.8% (95% CI, 33.2-50.4%), and 52.1% (95% CI, 42.0-62.2%) of the treated cohort after 1, 3, and 5 Hsu et al., 12 years of follow-up, respectively (Figure 2). The corresponding 1-, 3-, and 5-year cumulative incidences in the untreated cohort were 24.5% (95% CI, 21.4-27.5%), 54.3% (95% CI, 50.0-58.6%), and 63.9% (95% CI, 58.9-68.8%), respectively. Therefore, patients receiving postoperative anti-HCV regimen had a significantly lower recurrence rate (p=0.001). The unadjusted NNT associated with one fewer HCC recurrences after 1, 3, and 5 years were 12, 8, and 8, respectively (Table 2). The treated cohort also had a significantly lower mortality rate as compared with the untreated counterpart (p<0.001). The 1-, 3-, and 5-year cumulative incidences of mortality were 2.8% (95% CI, 0.4-5.2%), 10.8% (95% CI, 4.9-16.6%), and 15.4% (95% CI, 7.7-23.1%) in the treated patients, and 6.9% (95% CI, 5.1-8.7%), 24.8 (95% CI, 20.9-28.6%), and 47.0% (95% CI, 40.7-53.2%) in the untreated controls (Figure 3). The unadjusted NNT associated with one less mortality at 1, 3, and 5 years after antiviral treatment were 24, 7, and 3, respectively (Table 2). Multivariate-adjusted association of antiviral therapy with HCC recurrence The modified Cox proportional hazard model demonstrated that postoperative antiviral therapy was independently associated with 36% reduction in hazard of HCC recurrence (adjusted HR, 0.64; 95% CI, 0.50-0.83; p=0.001) (Table 3). Besides, major surgical resection as compared with a minor one (adjusted HR, 0.76; 95% CI, 0.59-0.97; p=0.027) and regular NSAID or aspirin use (adjusted HR, 0.80; 95% CI, 0.66-0.97; Hsu et al., 13 p=0.026) were also linked to reduction of recurrent HCC. Stratified analyses for recurrent HCC in association with antiviral therapy The association between postoperative antiviral therapy and reduced HCC recurrence was generally consistent across different patient subgroups, since the estimated HRs favored antiviral treatment in all strata (Figure 4). The reduction of hazard, however, differed in magnitude among patients according to age, liver cirrhosis, diabetes mellitus, and use of metformin. The association was more pronounced in patients younger (adjusted HR, 0.47; 95% CI, 0.30-0.73) versus older than 60 years (adjusted HR, 0.80; 95% CI, 0.59-1.09), without (adjusted HR, 0.56; 95% CI, 0.40-0.80) versus with cirrhosis (adjusted HR, 0.82; 95% CI, 0.56-1.20), and without (adjusted HR, 0.60; 95% CI, 0.45-0.81) versus with diabetes (adjusted HR, 0.86; 95% CI, 0.51-1.44). DISCUSSION This nationwide population-based study unraveled a significantly lower risk of recurrent HCC in CHC patients who treated their HCV infection postoperatively with peginterferon plus ribavirin, as compared with those whose CHC was left untreated. The hazard was reduced by 36% (adjusted HR 0.64; 95% CI, 0.50-0.83; p=0.001) after adjustment for possible confounding. The unadjusted NNT in association with one patient free of recurrent HCC at 1, 3, and 5 years after antiviral treatment were 12, Hsu et al., 14 8, and 8, respectively. Nevertheless, the magnitude of association appeared to differ among patient subgroups, in that the attenuated risk of HCC recurrence was more apparent in younger patients without cirrhosis or diabetes. These findings not only implicate that antiviral treatment may still ameliorate hepatocellular carcinogenesis even when HCV infection has progressed to the stage of HCC, but also characterize those who are more likely to benefit from this management. To date, there has been no adjuvant therapy approved for HCC after curative resection (6, 7). Conventional interferon alpha has been tested for this indication, but the results from randomized trials involving CHC patients were conflicting (27-30). Kubo et al. reported in a small trial (N=30) that postoperative administration of interferon for 2 years decreased recurrence of resected HCC (27), whereas Mazzaferro et al. concluded that adjuvant interferon for 48 weeks could not prevent HCC recurrence in 150 HCV-infected cirrhotic patients (28). The other two randomized trials, which recruited predominantly HBV-infected patients along with some CHC patients, also conflicted in the efficacy for secondary prevention of HCC (29, 30). In addition, therapeutic agent, dosing protocol, patient characteristic, and study endpoint also varied remarkably across these trials. Therefore, conventional interferon cannot be accepted as the standard care following HCC resection in CHC patients (7), despite a positive result from meta-analyses (31). Hsu et al., 15 Peginterferon alpha plus ribavirin has become the standard anti-HCV regimen for a decade (32, 33), but its efficacy in preventing recurrence of curatively treated HCC remains undetermined. Two previous studies addressing this issue did not find peginterferon-based therapy was associated with fewer recurrences (34, 35). In a cohort study consisting of 182 patients predominantly receiving radiofrequency ablation, Hagihara et al. reported HCC recurred similarly between 37 treated and 145 untreated patients (58% versus 70% at 5 years; p=0.17) (34). By taking a propensity score approach, Tanimoto et al. showed that recurrence did not differ between patients with and without postoperative peginterferon-based treatment (55.3% versus 44.7%; p=0.36; n=38 in both groups) (35). Both studies were probably underpowered because of the small number of participants. Besides, differences in demographics, HCC treatment, antiviral medication, outcome definition, and follow-up duration might also factor in the discrepancy between their results and ours. Based on our data, it needs a large sample comprising representative subgroups to uncover the association between postoperative antiviral treatment and HCC recurrence, in that the recurrence rate among treated patients may be lower but remains substantial and that certain patient characteristics can modify the association. Peg-interferon plus ribavirin is highly effective in achieving HCV eradication in Taiwan (36, 37), where a favorable genetic variation in IL28B is prevalent (38), and Hsu et al., 16 has been validated among Taiwanese patients with HCC in a multicenter trial (39). However, this study in and of itself could not delineate how virological response might have influenced the association. Because linking from the NHIRD to individual patients’ laboratory results was forbidden for privacy protection, we were unable to determine whether viral elimination mediated this association. Nevertheless, a large body of evidence has indicated that sustained virological response to antiviral treatment appears essential to reduce risk of developing HCV-related HCC (15,16). The large-scale randomized and placebo-controlled HALT-C trial has also refuted antitumor efficacy of peg-interferon in CHC patients who failed to eradicate HCV (40). In our opinion, antiviral efficacy was more likely than anti-proliferative property to account for the observed association in our study, although further research is clearly required to clarify the underlying mechanism. Furthermore, different viral clearance rate could plausibly explain why age, cirrhosis, and diabetes modified the association with recurrent HCC, since these were all validated host features predictive of therapeutic response (41-43). In consistence with our previous study which focused on recurrent HCC in patients with HBV infection (21), this study also uncovered an inverse association between use of aspirin or NSAID and risk of HCC recurrence. The mechanism of this intriguing finding may involve induction of cell cycle arrest and apoptosis in HCC cells (44, 45), and should inspire more investigation. Hsu et al., 17 This study has applied a number of methodological procedures to avoid a biased or confounded result, in addition to adjusting for multiple parameters in the multivariate analyses. First, enrollment was explicitly restricted to patients who could tolerate and recover from liver resection; whose performance status as well as hepatic reserve was therefore unlikely to contraindicate use of interferon and ribavirin. Second, in order to ensure comparability of the study cohorts, enrolled patients were matched in age, gender and cirrhosis. The treated and untreated cohorts were consequently similar in their baseline characteristics including the comorbidity index, i.e., the Charlson’s score. Moreover, matching in the time period from surgery to administration of antiviral therapy prevented the immortal time bias (23). Third, the universal coverage of Taiwan National Health Insurance, which fully reimbursed peginterferon and ribavirin for treating HCV infection, precluded healthcare accessibility or financial disparity as a determinant for receiving treatment or not. Last but not the least, we recognized how mortality might have confounded the estimation of the association with HCC recurrence (46). Since antiviral treatment could have affected survival by ameliorating the background liver disease, without relation to any effect on HCC, the higher mortality in the untreated patients would have overestimated their HCC recurrence rate and spuriously exaggerated the difference between the study cohorts, had the censoring caused by death been simply dismissed Hsu et al., 18 as non-informative (47, 48). Therefore, we believe these data valuable for physicians and surgeons who need to weigh up the benefits and risks of using peginterferon and ribavirin after resection of HCC, even though the observational nature of this study forestalled definite ascertainment of the causal relationship. Several limitations warrant discussion. First, lack of direct laboratory results in the Taiwan NHIRD prohibited exploration in terms of virological response, viral genotype, baseline viral load, size and number of tumors, and histological differentiation. Second, we were unable to determine the adverse reactions related to peginterferon and ribavirin. Nevertheless, nearly 90% of those who ever started postoperative antiviral therapy eventually completed a minimum of 16-week course, indicating tolerability of this regimen in these patients. Besides, a multicenter trial from Taiwan has confirmed its applicability in HCC patients (39). Finally, for the purpose of internal validity, we deliberately matched the postoperative period prior to antiviral treatment and restricted enrollment in patients free of recurrence within 3 months of surgery. Given that the time pattern of recurrence has been shown to correlate with its pathogenesis (49), recurrent HCC in this study might more likely result from de novo carcinogenesis instead of preexistent micro-metastasis. We accordingly suggest caution be exercised before extrapolating our findings in the setting of immediate recurrence following resection. Hsu et al., 19 In summary, recurrence of HCV-related HCC after surgical resection is reduced in patients who receive postoperative antiviral therapy with peginterferon plus ribavirin, as compared with those who never treat their HCV infection. Moreover, greater risk reduction of recurrent HCC is observed in younger patients (<60 years) and those without cirrhosis or diabetes. These results implicate that antiviral therapy appears better late than never in CHC patients with curable HCC. How to improve outcomes when the current therapy is either intolerable or ineffective warrants further research. ACKNOWLEDGEMENT This work is supported by Taiwan’s National Health Research Institutes (PH-101-PP-23) and based on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes. The interpretations and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health or the National Health Research Institutes. Citation of URL: http://www.nhri.org.tw/nhird/en/index.htm. Yao-Chun Hsu reports having received lecture fees from Merck Sharp & Dohme (Taiwan), Roche (Taiwan), and Bristol-Myers Squibb (Taiwan). There is no other potential conflict of interest to declare. Hsu et al., 20 REFERENCES 1. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology 2004;127:S27-34. 2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485-1491. 3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-2576. 4. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45:529-538. 5. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-1273 e1261. 6. Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 2009;10:1111-1118. 7. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-1022. Hsu et al., 21 8. Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg 1996;83:1219-1222. 9. Sasaki Y, Yamada T, Tanaka H, Ohigashi H, Eguchi H, Yano M, Ishikawa O, et al. Risk of recurrence in a long-term follow-up after surgery in 417 patients with hepatitis B- or hepatitis C-related hepatocellular carcinoma. Ann Surg 2006;244:771-780. 10. Ng IO, Guan XY, Poon RT, Fan ST, Lee JM. Determination of the molecular relationship between multiple tumour nodules in hepatocellular carcinoma differentiates multicentric origin from intrahepatic metastasis. J Pathol 2003;199:345-353. 11. Morimoto O, Nagano H, Sakon M, Fujiwara Y, Yamada T, Nakagawa H, Miyamoto A, et al. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J Hepatol 2003;39:215-221. 12. Camma C, Giunta M, Andreone P, Craxi A. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approach. J Hepatol 2001;34:593-602. 13. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, et al. Hsu et al., 22 Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521-1531. 14. Lin SM, Yu ML, Lee CM, Chien RN, Sheen IS, Chu CM, Liaw YF. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol 2007;46:45-52. 15. Yu ML, Lin SM, Chuang WL, Dai CY, Wang JH, Lu SN, Sheen IS, et al. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir Ther 2006;11:985-994. 16. Singal AK, Singh A, Jaganmohan S, Guturu P, Mummadi R, Kuo YF, Sood GK. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol 2010;8:192-199. 17. Makiyama A, Itoh Y, Kasahara A, Imai Y, Kawata S, Yoshioka K, Tsubouchi H, et al. Characteristics of patients with chronic hepatitis C who develop hepatocellular carcinoma after a sustained response to interferon therapy. Cancer 2004;101:1616-1622. 18. Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335-1374. 19. Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT. Effective reduction of Hsu et al., 23 gastric cancer risk with regular use of nonsteroidal anti-inflammatory drugs in Helicobacter pylori-infected patients. J Clin Oncol 2010;28:2952-2957. 20. Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, Lin JH, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut 2012 (in press). 21. Wu CY, Chen YJ, Ho JH, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma following liver resection. JAMA 2012, 14;308(18):1906-14. 22. Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1994;271:1598-1601. 23. Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol 2008;19:841-843. 24. Simons JP, Ng SC, Hill JS, Shah SA, Zhou Z, Tseng JF. In-hospital mortality from liver resection for hepatocellular carcinoma: a simple risk score. Cancer 2010;116:1733-1738. 25. Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 16:1141-1154, 1988. 26. Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a Hsu et al., 24 competing risk. JASA 94:496-509, 1999. 27. Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Yamazaki O, Shiomi S, et al. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. A randomized, controlled trial. Ann Intern Med 2001;134:963-967. 28. Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology 2006;44:1543-1554. 29. Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, Ng IO, Fan ST, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg 2007;245:831-842. 30. Chen LT, Chen MF, Li LA, Lee PH, Jeng LB, Lin DY, Wu CC, et al. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann Surg 2012;255:8-17. 31. Miyake Y, Takaki A, Iwasaki Y, Yamamoto K. Meta-analysis: interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J Viral Hepat 2010;17:287-292. 32. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Hsu et al., 25 Goodman ZD, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958-965. 33. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr., Haussinger D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-982. 34. Hagihara H, Nouso K, Kobayashi Y, Iwasaki Y, Nakamura S, Kuwaki K, Toshimori J, et al. Effect of pegylated interferon therapy on intrahepatic recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. Int J Clin Oncol 2011;16:210-220. 35. Tanimoto Y, Tashiro H, Aikata H, Amano H, Oshita A, Kobayashi T, Kuroda S, et al. Impact of pegylated interferon therapy on outcomes of patients with hepatitis C virus-related hepatocellular carcinoma after curative hepatic resection. Ann Surg Oncol 2012;19:418-425. 36. Yu ML, Dai CY, Huang JF, Hou NJ, Lee LP, Hsieh MY, et al. A randomised study of peginterferon and ribavirin for 16 versus 24 weeks in patients with genotype 2 chronic hepatitis C. Gut. 2007;56(4):553-9. 37. Liu CH, Liu CJ, Lin CL, Liang CC, Hsu SJ, Yang SS, et al. Pegylated interferon-alpha-2a plus ribavirin for treatment-naive Asian patients with hepatitis C Hsu et al., 26 virus genotype 1 infection: a multicenter, randomized controlled trial. Clin Infect Dis. 2008;47(10):1260-9. 38. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399-401. 39. Huang JF, Yu ML, Huang CF, Chiu CF, Dai CY, Huang CI, Yeh ML, et al. The efficacy and safety of pegylated interferon plus ribavirin combination therapy in chronic hepatitis c patients with hepatocellular carcinoma post curative therapies - a multicenter prospective trial. J Hepatol 2011;54:219-226. 40. Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, et al. N Engl J Med 2008; 359:2429-2441 41. Hadziyannis SJ, Sette H, Jr., Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140:346-355. 42. Huang CF, Yang JF, Dai CY, Huang JF, Hou NJ, Hsieh MY, Lin ZY, et al. Efficacy and safety of pegylated interferon combined with ribavirin for the treatment of older patients with chronic hepatitis C. J Infect Dis 2010;201:751-759. 43. Romero-Gomez M, Del Mar Viloria M, Andrade RJ, Salmeron J, Diago M, Hsu et al., 27 Fernandez-Rodriguez CM, Corpas R, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology 2005;128:636-641. 44. Raza H, John A, Benedict S. Acetylsalicylic acidinduced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur J Pharmacol. 2011;668(1-2):15-24. 45. Hossain MA, Kim DH, Jang JY, Kang YJ, Yoon JH, Moon JO, Chung HY, et al. Aspirin induces apoptosis in vitro and inhibits tumor growth of human hepatocellular carcinoma cells in a nude mouse xenograft model. Int J Oncol. 2012;40(4):1298-1304. 46. Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698-711. 47. Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer 2004;91:1229-1235. 48. Hsu YC, Lin JT, Chen TT, Wu MS, Wu CY. Long-term risk of recurrent peptic ulcer bleeding in patients with liver cirrhosis: A 10-year nationwide cohort study. Hepatology 2012;56:698-705. 49. Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, Huo TI, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. Hsu et al., 28 2009;51(5):890-897. FIGURE LEGENDS Figure 1. Flow diagram of identification and enrollment of the study subjects; CHC, chronic hepatitis C; HCC, hepatocellular carcinoma Figure 2. Recurrence of surgically resected hepatocellular carcinoma in patients treated with postoperative antiviral therapy (treated cohort, blue line) and matched controls without antiviral treatment (untreated cohort, red line); death prior to recurrence is adjusted as a competing cause of risk. Figure 3. The mortality rates between the treated (blue line) and untreated cohorts (red line). Figure 4. Multivariate stratified analyses for the association between postoperative antiviral therapy and recurrence of hepatocellular carcinoma after resection. All subgroup analyses are adjusted for confounders and accounted for death as the competing cause of risk. CI, confidence interval; HR, hazard ratio; NSAID, Hsu et al., 29 non-steroidal anti-inflammatory drug