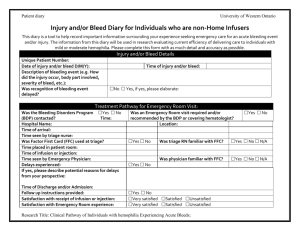
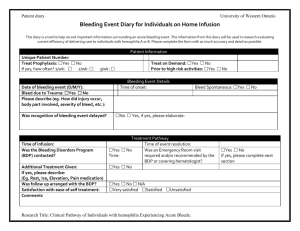
Antihemophilic Factor and Clotting Factors
advertisement

REVIEW REQUEST FOR Antihemophilic Factor and Clotting Factors Provider Data Collection Tool Based on Medical Policy DRUG.00066 Policy Last Review Date: 11/05/2015 Policy Effective Date: 11/09/2015 Request Date: / / Initial Authorization Request Buy and bill Provider Tool Effective Date: Subsequent Request Individual’s Name: Date of Birth: / / Individual’s Phone Number: Insurance Identification Number: Primary Diagnosis: 11/09/2015 Diagnosis Code(s) (if known): Ordering Provider Name & Specialty: Individual’s Weight (lbs) (kg) Individual’s Height (in) (cm) Provider ID Number: Office Address: Contact Name and Office Phone Number: Office Fax Number: Servicing Provider Name & Specialty (If different than Ordering Provider): Provider ID Number: Office Address: Contact Name and Office Phone Number: Place of Service: Home Office Dialysis Center Ambulatory Infusion Ambulatory Infusion Center Drug Name/HCPCS Code (if known) Advate J7192 Kogenate-FS J7192 Alphanate J7186 Monoclate-P J7190 AlphaNine SD J7193 Mononine J7193 Alprolix J7201 Novoeight J7182 Bebulin VH J7194 NovoSeven RT J7189 Benefix J7195 Nuwiq J7192 Coagadex J7199 (eff 1/1/16) Profilnine SD J7194 Corifact J7180 J7181 Recombinate J7192 Eloctate J7205 (eff 1/1/16) RiaSTAP J7178 Eloctate Q9975 (delt 12/31/15) Rixubis J7200 FEIBA NF J7198 Tretten J7180 J7181 Helixate-FS J7192 Wilate J7183 Hemofil M J7190 Xyntha J7185 Humate-P J7187 Ixinity J7195 Koate DVI J7190 Obizur J7191 (delt 12/31/15) J7188 (eff 1/1/16) Other: When did the individual first start this drug? / / Duration: (Weeks) Office Fax Number: Outpatient Hospital Other: Dose to be administered: (I.U./kg) (Other) Frequency (Days, Wks, Months) Start Date For This Request: / / This provider tool addresses requests for select hemophilia and clotting factor replacement treatments created from blood products (human plasma-derived) and others that are manufactured (recombinant). The provider tool does not address requests for fibrin products, fibrin sealants and blood products provided by blood banks. Replacement therapy may be given on a routine, preventive basis which is also called prophylactic therapy. The infusion of factor replacements given to stop a bleeding episode is called demand therapy. Please identify and complete the appropriate section for this individual. Check all that apply. A. Anti-inhibitor Coagulant Complex (FEIBA NF) Anti-inhibitor coagulant complex (FEIBA NF) is requested to treat individual diagnosed with hemophilia A or B with inhibitors to Factor VIII or Factor IX FEIBA NF will be given to treat bleeding episodes. (If checked, mark the following when it applies) The individual’s bleeding episodes are NOT a result of coagulation factor deficiencies in the absence of inhibitors to coagulation Factor VIII or coagulation Factor IX. FEIBA NF will be given as peri-procedural management for surgical, invasive or interventional radiology procedures FEIBA NF will be given as routine prophylaxis to prevent or reduce the frequency of bleeding episodes B. Factor VIIa Recombinant (NovoSeven RT) Recombinant coagulation Factor VIIa (NovoSeven RT) is requested for treatment of bleeding episodes (If checked answer the following) Individual has hemophilia A or B with inhibitors to Factor VIII or Factor IX Individual has acquired hemophilia Individual has congenital Factor VII deficiency. Recombinant coagulation Factor VIIa is requested as prevention of bleeding in surgical interventions or invasive procedures (If checked answer the following) Individual has hemophilia A or B with inhibitors to Factor VIII or Factor IX Individual has acquired hemophilia Individual has congenital Factor VII deficiency Recombinant coagulation Factor VIIa is requested for treatment of bleeding episodes and peri-operative management in individuals with Glanzmann’s thrombasthenia and a documented refractoriness to platelet transfusions with or without antibodies to Platelets Other ______________________ C. Antihemophilic factor (factor VIII) Human plasma-derived (Hemofil M, Koate-DVI, Monoclate-P) Human plasma-derived antihemophilic Factor VIII (Hemofil M, Koate-DVI, Monoclate-P) is requested for treatment of bleeding episodes in an individual with hemophilia A and factor VIII deficiency Human plasma-derived antihemophilic Factor VIII (Koate-DVI, Monoclate-P) is requested for peri-procedural management for surgical, invasive or interventional radiology procedures in an individual with hemophilia A and factor VIII deficiency Human plasma-derived antihemophilic Factor VIII (Hemofil, Koate-DVI, Monoclate-P) is requested for routine prophylaxis to prevent or reduce the frequency of bleeding episodes (If checked, answer the following) Individual has severe hemophilia A (defined as less than or equal to 1 International Unit per deciliter [IU/dL] or 1% endogenous Factor VIII) Individual has mild to moderate hemophilia A (defined as endogenous Factor VIII less than or equal to 40 IU/dL [less than or equal to 40%], but greater than 1 IU) (IF checked, answer the following that apply) Individual has a history of : 1 or more episodes of spontaneous bleeding into joint 1 or more episodes of spontaneous bleeding into the central nervous system 4 or more episodes of soft tissue bleeding in an 8 week period Human plasma-derived antihemophilic Factor VIII will NOT be given to treat an individual with von Willebrand disease (VWD) Other ______________________ Page 2 of 7 D. Antihemophilic factor (factor VIII) Recombinant (Advate, Helixate FS, Kogenate FS, Novoeight, Nuwiq. Recombinate, Xyntha) Request is for Antihemophilic Factor VIII Recombinant (Advate, Helixate FS, Kogenate FS, Novoeight, Nuwiq, Recombinate, Xyntha) treatment (If checked, answer the following uses that apply to the individual) Treatment of bleeding episodes resulting from hemophilia A and factor VIII deficiency. Peri-procedural management for surgical, invasive or interventional radiology procedures in an individual with hemophilia A and factor VIII deficiency Antihemophilic Factor VIII Recombinant (Advate, Helixate FS, Novoeight, Nuwiq) is requested as routine prophylaxis to prevent or reduce the frequency of bleeding episodes (If checked, answer the following) Individual has severe hemophilia A (defined as less than or equal to 1 International Unit per deciliter [IU/dL] or 1% endogenous Factor VIII) Individual has mild to moderate hemophilia A (defined as endogenous Factor VIII less than or equal to 40 IU/dL [less than or equal to 40%], but greater than 1 IU) (If checked, mark the following as they apply) The individual has a documented history of: 1 or more episodes of spontaneous bleeding into joint 1 or more episodes of spontaneous bleeding into the central nervous system 4 or more episodes of soft tissue bleeding in an 8 week period Antihemophilic Factor VIII Recombinant (Helixate FS, Kogenate FS) is requested as routine prophylaxis for a child (age 0-16 years) with hemophilia A and factor VIII deficiency to reduce the risk of joint damage in those without pre-existing joint damage Antihemophilic Factor VIII Recombinant (Recombinate) is requested as treatment of an individual with acquired Factor VIII inhibitors not exceeding 10 Bethesda Unit (BU) per milliliter (mL). Antihemophilic Factor VIII Recombinant (Advate, Helixate FS, Kogenate FS, Novoeight, Nuwiq, Recombinate, Xyntha) will NOT be given to treat an individual with von Willebrand disease (VWD) Other ______________________ E. Recombinant Antihemophilic Factor, Fc Fusion Protein (Eloctate) Recombinant antihemophilic factor, Fc fusion protein ([rFVIIIFc]) (Eloctate) is requested for an individual with severe hemophilia A (congenital Factor VIII deficiency) (If checked, answer the following) Individual has less than or equal to 1 International Unit per deciliter (IU/dL) (less than or equal to 1%) endogenous factor VIII Eloctate is planned for control and prevention of acute bleeding episodes Eloctate is planned for peri-procedural management for surgical, invasive or interventional radiology procedures Eloctate is planned for routine prophylaxis to prevent or reduce the frequency of bleeding episodes Eloctate is requested for an individual with mild to moderate hemophilia A (congenital Factor VIII deficiency) (If checked, answer the following) Individual has endogenous factor VIII level less than 40 IU/dl (less than or equal to 40%) but greater than 1 IU/dl Eloctate is planned for control and prevention of acute bleeding episodes Eloctate is planned for peri-procedural management for surgical, invasive or interventional radiology procedures Eloctate is planned for routine prophylaxis to prevent or reduce the frequency of bleeding episodes (If checked, answer the following) The individual has a documented history of: 1 or more episodes of spontaneous bleeding into joint 1 or more episodes of spontaneous bleeding into the central nervous system 4 or more episodes of soft tissue bleeding in an 8 week period Eloctate will NOT be given to treat an individual with von Willebrand disease (VWD) Other ______________________ Page 3 of 7 F. Antihemophilic Factor (Recombinant), Porcine Sequence (Obizur) Antihemophilic Factor (Recombinant), Porcine Sequence is requested for treatment of bleeding episodes in an adult with acquired hemophilia A. Antihemophilic Factor (Recombinant), Porcine Sequence is requested for: (check the following that apply ) Treatment an individual with congenital hemophilia A with Factor VIII deficiency Treatment an individual with von Willebrand disease Treatment of an individual with acquired hemophilia A AND baseline anti-porcine Factor VIII inhibitor titer greater than 20 BU/mL Other ______________________ G. Antihemophilic Factor VIII/von Willebrand Factor Complex (Alphanate, Humate-P, Wilate) Antihemophilic Factor VIII/von Willebrand Factor Complex (Alphanate, Humate-P, Wilate) is being requested as a treatment for an individual with von Willebrand disease (VWD) (Type 1, 2 or unknown). (If checked, answer the following) VWD is severe VWD is mild to moderate AND use of desmopressin is known or suspected to be inadequate Individual is being treated for spontaneous or trauma-induced bleeding episodes Individual’s treatment is for peri-procedural management for surgical, invasive or interventional radiology procedures. Antihemophilic Factor VIII/von Willebrand Factor Complex (Alphanate, Humate-P) is requested for treatment of bleeding episodes in an individual with hemophilia A and Factor VIII deficiency Antihemophilic Factor VIII/von Willebrand Factor Complex (Alphanate) is being requested for treatment of bleeding episodes in an individual with acquired Factor VIII deficiency. Antihemophilic Factor/von Willebrand Factor Complex (Alphanate, Humate-P, Wilate) is NOT being used for prophylaxis therapy for an individual with VWD. Antihemophilic Factor/von Willebrand Factor Complex (Alphanate) is NOT being used for an individual with severe VWD (Type 3) undergoing major surgery. Antihemophilic Factor/von Willebrand Factor Complex (Wilate) is NOT being used for an individual with hemophilia A. Other ______________________ H. Coagulation Factor IX, Human plasma-derived (Alphanine SD, Mononine) The request is for Human plasma-derived coagulation Factor IX (Alphanine SD, Mononine) to treat an individual with hemophilia B and Factor IX deficiency The request is for Human plasma-derived coagulation Factor IX (Alphanine SD, Mononine) as routine prophylaxis to prevent or reduce the frequency of bleeding episodes (If checked, answer the following that apply to the individual) Individual has severe hemophilia B (defined as less than or equal to 1 IU/dL or 1% endogenous Factor IX); Individual has mild to moderate hemophilia B (defined as endogenous Factor IX less than or equal to 40 IU/dL [less than 40% ], but greater than 1 IU/dL) (If checked, answer the following that apply) The individual has a documented history of: 1 or more episodes of spontaneous bleeding into joint 1 or more episodes of spontaneous bleeding into the central nervous system 4 or more episodes of soft tissue bleeding in an 8 week period Human plasma-derived coagulation Factor IX (Alphanine SD, Mononine) will NOT be used to treat or reverse coumarin-induced anticoagulation Human plasma-derived coagulation Factor IX (Alphanine SD, Mononine) will NOT be used to treat an individual in a hemorrhagic state or with coagulopathy associated with liver dysfunction Human plasma-derived coagulation Factor IX (Alphanine SD, Mononine) will NOT be used to treat an individual with hemophilia A with inhibitors to factor VIII Human plasma-derived coagulation Factor IX (Alphanine SD, Mononine) will NOT be used as replacement therapy for other clotting factors which include factors II, VII and X Other ______________________ Page 4 of 7 I. J. Factor IX Complex, Human plasma-derived (Bebulin, Profilnine SD) The request is for Human plasma-derived Factor IX complex (Bebulin, Profilnine SD) to treat bleeding episodes in an individual diagnosed with hemophilia B (congenital factor IX deficiency or Christmas disease). The request is for Human plasma-derived Factor IX (Bebulin, Profilnine SD) as routine prophylaxis to prevent or reduce the frequency of bleeding episodes (If checked, answer the following) Individual has severe hemophilia B (defined as less than or equal to 1 IU/dL or 1% endogenous Factor IX); Individual has mild to moderate hemophilia B (defined as endogenous Factor IX less than or equal to 40 IU/dL [less than 40% ], but greater than 1 IU/dL) (If checked, answer the following that apply) The individual has a documented history of: 1 or more episodes of spontaneous bleeding into joint 1 or more episodes of spontaneous bleeding into the central nervous system 4 or more episodes of soft tissue bleeding in an 8 week period Human plasma-derived Factor IX (Bebulin, Profilnine SD) will NOT be used to treat an individual diagnosed with Factor VII deficiency Other ______________________ Factor IX Recombinant (Benefix, Ixinity, Rixubis) Request is for Recombinant coagulation Factor IX (Benefix, Rixubis) to treat an individual with hemophilia B (congenital factor IX deficiency or Christmas disease). (If checked, answer the following) The agent is to treat bleeding episodes The agent is for peri-procedural management for surgical, invasive or interventional radiology procedures The request is for Recombinant coagulation Factor IX (Benefix, Rixubis) for routine prophylaxis to prevent or reduce the frequency of bleeding episodes. (If checked, answer the following) Individual has severe hemophilia B (defined as less than or equal to 1 IU/dL or 1% endogenous Factor IX) Individual has mild to moderate hemophilia B (defined as endogenous Factor IX less than or equal to 40 IU/dL [less than 40% ], but greater than 1 IU/dL) (If checked, answer the following that apply) The individual has a documented history of: 1 or more episodes of spontaneous bleeding into joint 1 or more episodes of spontaneous bleeding into the central nervous system 4 or more episodes of soft tissue bleeding in an 8 week period Request is for recombinant coagulation Factor IX (Ixinity) to treat an individual age 12 years or older with hemophilia B (congenital factor IX deficiency or Christmas disease) (If checked, answer the following) Ixinity will be used to treat bleeding episodes Ixinity will be used for peri-procedural management for surgical, invasive or interventional radiology procedures Ixinity will be used for routine prophylaxis to prevent or reduce the frequency of bleeding episodes (If checked, answer the following) Individual has severe hemophilia B (defined as less than or equal to 1 IU/dL or 1% endogenous Factor IX); Individual has mild to moderate hemophilia B (defined as endogenous Factor IX less than or equal to 40 IU/dL [less than 40% ], but greater than 1 IU/dL) (If checked, answer the following that apply) The individual has a documented history of: 1 or more episodes of spontaneous bleeding into joint 1 or more episodes of spontaneous bleeding into the central nervous system 4 or more episodes of soft tissue bleeding in an 8 week period Recombinant coagulation Factor IX (Benefix, Ixinity, Rixubis ) will NOT be used to treat an individual with other factor deficiencies (for example, factors II, VII, VIII and X) Recombinant coagulation Factor IX (Benefix, Ixinity, Rixubis ) will NOT be used to treat an individual with hemophilia A with inhibitors to factor VIII Recombinant coagulation Factor IX (Benefix, Ixinity, Rixubis ) will NOT be used to reverse coumarin-induced anticoagulation Recombinant coagulation Factor IX (Benefix, Ixinity, Rixubis ) will NOT be used to treat bleeding due to low levels of liver-dependent coagulation factors Recombinant coagulation Factor IX (Benefix, Ixinity, Rixubis) will NOT be used to induce immune tolerance in an individual with hemophilia B. Other ______________________ Page 5 of 7 K. Recombinant Coagulation Factor IX, Fc Fusion Protein (Alprolix™) Request is for recombinant coagulation Factor IX, Fc Fusion [rFIXFc] (Alprolix™ ) to treat an individual with severe hemophilia B (congenital Factor IX deficiency) (If checked answer the following) Individual has less than or equal to 1 International Unit per deciliter (IU/dl) (less than or equal to 1%) endogenous factor IX Alprolix™ is planned for treatment of bleeding episodes Alprolix™ is planned for peri-procedural management for surgical, invasive or interventional radiology procedures Alprolix™ is planned for routine prophylaxis to prevent or reduce the frequency of bleeding episodes Request is for recombinant coagulation Factor IX, Fc Fusion [rFIXFc] (Alprolix™ ) to treat an individual with mild to moderate hemophilia B (congenital factor IX deficiency) (If checked answer the following as they apply to the individual) Individual has endogenous factor IX level less than 40 International Units per deciliter (IU/dl) (less than or equal to 40%) but greater than 1 IU/dl Alprolix™ is planned for treatment of bleeding disorders Alprolix™ is planned for peri-procedural management for surgical, invasive or interventional radiology procedures Alprolix™ is planned for routine prophylaxis to prevent or reduce the frequency of bleeding episodes The individual has a documented history of: 1 or more episodes of spontaneous bleeding into joint 1 or more episodes of spontaneous bleeding into the central nervous system 4 or more episodes of soft tissue bleeding in an 8 week period Alprolix™ will NOT be used to induce immune tolerance in an individual with hemophilia B Other ______________________ L. Coagulation Factor X Human plasma- derived (Coagadex®) Request is for human plasma derived coagulation Factor X (Coagadex) for individual is age 12 years or older (If checked, mark all of the following that apply to the individual) Individual has severe or moderate hereditary Factor X deficiency (defined as less than 5 International Unit per deciliter (IU/dl) or 5% endogenous Factor X) Coagadex® will be used for the treatment of bleeding episodes Coagadex® will NOT be used for perioperative management of bleeding in major surgery Individual has mild hereditary Factor X deficiency (defined as greater than or equal to 5 International Unit per deciliter (IU/dl) or 5% endogenous Factor X) Coagadex® will be used for peri-procedural management for surgical, invasive or interventional radiology procedures. Other: _____________________________________ M. Factor XIII (Corifact, Tretten) Human plasma-derived concentrate Factor XIII (Corifact) is to be given to an individual diagnosed with Factor XIII deficiency (If checked, identify the purpose of the treatment) As routine prophylactic treatment to prevent or reduce the frequency of bleeding episodes For peri-procedural management for surgical, invasive or interventional radiology procedures Recombinant coagulation Factor XIII A-Subunit (Tretten) is to be given to an individual with congenital Factor XIII A-subunit deficiency for routine prophylaxis of bleeding Coagulation Factor XIII (Corifact, Tretten) will NOT be used to treat an an individual with congenital Factor XIII B-subunit deficiency Other ______________________ N. Fibrinogen Concentrate, Human plasma-derived (RiaSTAP) Human plasma-derived fibrinogen concentrate is requested for treatment of acute bleeding episodes as a result of congenital fibrinogen deficiency (that is, afibrinogenemia or hypofibrinogenemia). Human plasma-derived fibrinogen concentrate will NOT be given to treat an individual with dysfibrinogenemia Other ______________________ O. Other uses not specified above. Please specify: (Please submit all supporting documents including labs, progress notes, imaging, etc., for review.) Page 6 of 7 This request is being submitted: Pre-Claim Post–Claim. If checked, please attach the claim or indicate the claim number I attest the information provided is true and accurate to the best of my knowledge. I understand that the health plan or its designees may perform a routine audit and request the medical documentation to verify the accuracy of the information reported on this form. / / Name & Title of Provider or Provider Representative Completing Form Date & attestation (Please Print)* *The attestation fields must be completed by a provider or provider representative in order for the tool to be accepted Anthem UM Services, Inc., a separate company, is the licensed utilization review agent that performs utilization management services on behalf of your health benefit plan or the administrator of your health benefit plan. Page 7 of 7