Supplementary Table 12 - Word file (75 KB )
advertisement
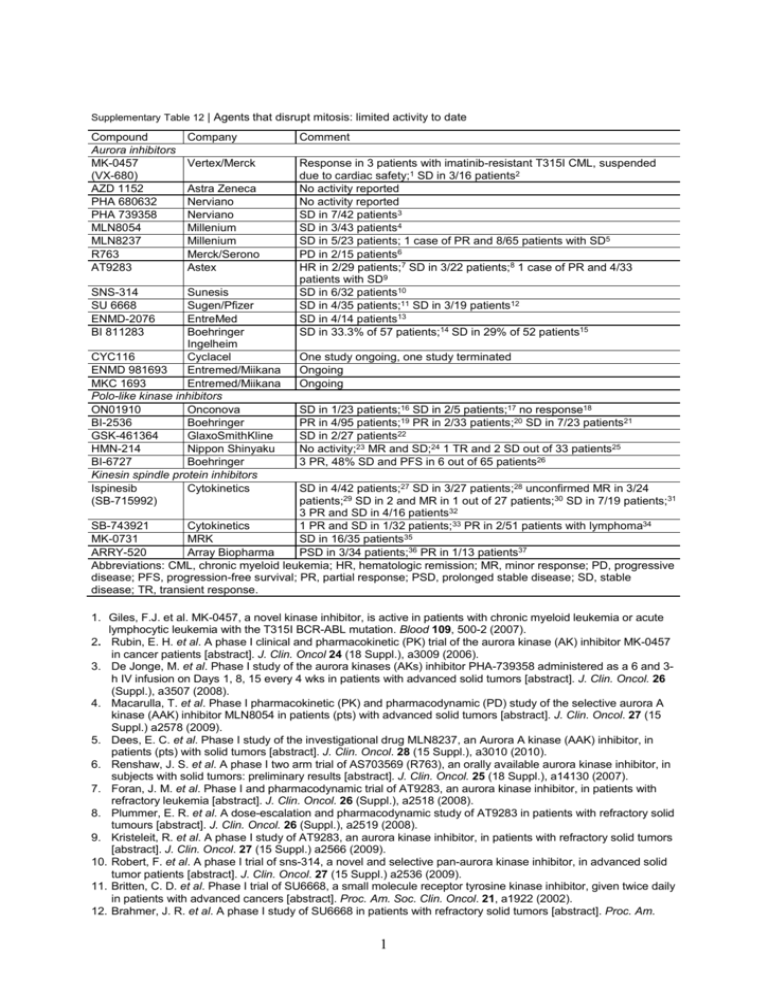
Supplementary Table 12 | Agents that disrupt mitosis: limited activity to date Compound Aurora inhibitors MK-0457 (VX-680) AZD 1152 PHA 680632 PHA 739358 MLN8054 MLN8237 R763 AT9283 SNS-314 SU 6668 ENMD-2076 BI 811283 Company Comment Vertex/Merck Response in 3 patients with imatinib-resistant T315I CML, suspended due to cardiac safety;1 SD in 3/16 patients2 No activity reported No activity reported SD in 7/42 patients3 SD in 3/43 patients4 SD in 5/23 patients; 1 case of PR and 8/65 patients with SD5 PD in 2/15 patients6 HR in 2/29 patients;7 SD in 3/22 patients;8 1 case of PR and 4/33 patients with SD9 SD in 6/32 patients10 SD in 4/35 patients;11 SD in 3/19 patients12 SD in 4/14 patients13 SD in 33.3% of 57 patients;14 SD in 29% of 52 patients15 Astra Zeneca Nerviano Nerviano Millenium Millenium Merck/Serono Astex Sunesis Sugen/Pfizer EntreMed Boehringer Ingelheim CYC116 Cyclacel ENMD 981693 Entremed/Miikana MKC 1693 Entremed/Miikana Polo-like kinase inhibitors ON01910 Onconova BI-2536 Boehringer GSK-461364 GlaxoSmithKline HMN-214 Nippon Shinyaku BI-6727 Boehringer Kinesin spindle protein inhibitors Ispinesib Cytokinetics (SB-715992) One study ongoing, one study terminated Ongoing Ongoing SD in 1/23 patients;16 SD in 2/5 patients;17 no response18 PR in 4/95 patients;19 PR in 2/33 patients;20 SD in 7/23 patients21 SD in 2/27 patients22 No activity;23 MR and SD;24 1 TR and 2 SD out of 33 patients25 3 PR, 48% SD and PFS in 6 out of 65 patients26 SD in 4/42 patients;27 SD in 3/27 patients;28 unconfirmed MR in 3/24 patients;29 SD in 2 and MR in 1 out of 27 patients;30 SD in 7/19 patients;31 3 PR and SD in 4/16 patients32 SB-743921 Cytokinetics 1 PR and SD in 1/32 patients;33 PR in 2/51 patients with lymphoma34 MK-0731 MRK SD in 16/35 patients35 ARRY-520 Array Biopharma PSD in 3/34 patients;36 PR in 1/13 patients37 Abbreviations: CML, chronic myeloid leukemia; HR, hematologic remission; MR, minor response; PD, progressive disease; PFS, progression-free survival; PR, partial response; PSD, prolonged stable disease; SD, stable disease; TR, transient response. 1. Giles, F.J. et al. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL mutation. Blood 109, 500-2 (2007). 2. Rubin, E. H. et al. A phase I clinical and pharmacokinetic (PK) trial of the aurora kinase (AK) inhibitor MK-0457 in cancer patients [abstract]. J. Clin. Oncol 24 (18 Suppl.), a3009 (2006). 3. De Jonge, M. et al. Phase I study of the aurora kinases (AKs) inhibitor PHA-739358 administered as a 6 and 3h IV infusion on Days 1, 8, 15 every 4 wks in patients with advanced solid tumors [abstract]. J. Clin. Oncol. 26 (Suppl.), a3507 (2008). 4. Macarulla, T. et al. Phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of the selective aurora A kinase (AAK) inhibitor MLN8054 in patients (pts) with advanced solid tumors [abstract]. J. Clin. Oncol. 27 (15 Suppl.) a2578 (2009). 5. Dees, E. C. et al. Phase I study of the investigational drug MLN8237, an Aurora A kinase (AAK) inhibitor, in patients (pts) with solid tumors [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a3010 (2010). 6. Renshaw, J. S. et al. A phase I two arm trial of AS703569 (R763), an orally available aurora kinase inhibitor, in subjects with solid tumors: preliminary results [abstract]. J. Clin. Oncol. 25 (18 Suppl.), a14130 (2007). 7. Foran, J. M. et al. Phase I and pharmacodynamic trial of AT9283, an aurora kinase inhibitor, in patients with refractory leukemia [abstract]. J. Clin. Oncol. 26 (Suppl.), a2518 (2008). 8. Plummer, E. R. et al. A dose-escalation and pharmacodynamic study of AT9283 in patients with refractory solid tumours [abstract]. J. Clin. Oncol. 26 (Suppl.), a2519 (2008). 9. Kristeleit, R. et al. A phase I study of AT9283, an aurora kinase inhibitor, in patients with refractory solid tumors [abstract]. J. Clin. Oncol. 27 (15 Suppl.) a2566 (2009). 10. Robert, F. et al. A phase I trial of sns-314, a novel and selective pan-aurora kinase inhibitor, in advanced solid tumor patients [abstract]. J. Clin. Oncol. 27 (15 Suppl.) a2536 (2009). 11. Britten, C. D. et al. Phase I trial of SU6668, a small molecule receptor tyrosine kinase inhibitor, given twice daily in patients with advanced cancers [abstract]. Proc. Am. Soc. Clin. Oncol. 21, a1922 (2002). 12. Brahmer, J. R. et al. A phase I study of SU6668 in patients with refractory solid tumors [abstract]. Proc. Am. 1 Soc. Clin. Oncol. 21, a335 (2002). 13. Bastos, B. R. et al. An open-label, dose escalation, safety, and pharmacokinetic study of ENMD-2076 administered orally to patients with advanced cancer [abstract]. J. Clin. Oncol. 27 (15 Suppl.) a3520 (2009). 14. Mross, K. B. et al. A phase I dose-escalation study of BI 811283, an Aurora B inhibitor, administered every three weeks in patients with advanced solid tumors [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a3011 (2010). 15. Scheulen, M. E. et al. A phase I dose-escalation study of BI 811283, an Aurora B inhibitor, administered days 1 and 15, every four weeks in patients with advanced solid tumors [abstract]. J. Clin. Oncol. 28 (Suppl.), e13065 (2010). 16. Vainshtein, J. M. et al. Phase I study of ON 01910.Na, a novel polo-like kinase 1 pathway modulator, administered as a weekly 24-hour continuous infusion in patients with advanced cancer [abstract]. J. Clin. Oncol. 26 (Suppl.), a2515 (2008). 17. Ohnuma, T. et al. Phase I study of ON 01910.Na by 3-day continuous infusion (CI) in patients (pts) with advanced cancer [abstract]. J. Clin. Oncol. 24 (18 Suppl.), a13137 (2006). 18. Donehower, R. C. et al. Phase I study of ON-01910.Na, a novel cell cycle inhibitor in adult patients with solid tumors [abstract]. J. Clin. Oncol. 24 (18 Suppl.), a13026 (2006). 19. Von Pawel, J. et al. Randomized phase II trial of two dosing schedules of BI 2536, a novel Plk-1 inhibitor, in patients with relapsed advanced or metastatic non-small-cell lung cancer (NSCLC) [abstract]. J. Clin. Oncol. 26 (Suppl.), a8030 (2008). 20. Ellis, P. M. et al. A phase I dose escalation trial of BI 2536, a novel Plk1 inhibitor, with standard dose pemetrexed in previously treated advanced or metastatic non-small cell lung cancer (NSCLC) [abstract]. J. Clin. Oncol. 26 (Suppl.), a8115 (2008). 21. Gandhi, L. et al. An open label phase II trial of the Plk1 inhibitor BI 2536, in patients with sensitive relapse small cell lung cancer (SCLC) [abstract]. J. Clin. Oncol. 27 (15 Suppl.) a8108 (2009). 22. Olmos, D. et al. Phase I first-in-human study of the polo-like kinase-1 selective inhibitor, GSK461364, in patients with advanced solid tumors [abstract]. J. Clin. Oncol. 27 (15 Suppl.) a3536 (2009). 23. Taylor, C., Dragovich, T., Simpson, A. & Von Hoff, D. A phase I and pharmacokinetic study of HMN-214 administered orally for 21 consecutive days, repeated every 28 days to patients with advanced solid tumors [abstract]. Proc. Am. Soc. Clin. Oncol. 21, a419 (2002). 24. Patnaik, A. et al. A phase I and pharmacokinetic (PK) study of HMN-214, an oral antimicrotubular agent with polo-like- and cyclin-dependent kinase inhibitory activities [abstract]. Proc. Am. Soc. Clin. Oncol. 21, a418 (2002). 25. Von Hoff, D. D. et al. A Phase I and pharmacokinetic study of HMN-214, a novel oral polo-like kinase inhibitor, in patients with advanced solid tumors [abstract]. J. Clin. Oncol. 22 (14 Suppl.), a3034 (2004). 26. Gil, T. et al. Final analysis of a phase I single dose-escalation study of the novel polo-like kinase 1 inhibitor BI 6727 in patients with advanced solid tumors [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a3061 (2010). 27. Chu, Q. S. et al. Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV Q 21 days [abstract]. J. Clin. Oncol. 22 (14 Suppl.), a2078 (2004). 28. Burris, H. A. et al. Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV days 1, 8, 15 q 28 days [abstract]. J. Clin. Oncol. 22 (14 Suppl.), a2004 (2004). 29. Jones, F. S. et al. Phase I study of ispinesib in combination with carboplatin in patients with advanced solid tumors [abstract]. J. Clin. Oncol 24 (18 Suppl.), a2027 (2006). 30. Heath, E. I. et al. A phase I dose escalation trial of ispinesib (SB-715992) administered days 1–3 of a 21-day cycle in patients with advanced solid tumors [abstract]. J. Clin. Oncol 24 (18 Suppl.), a2026 (2006). 31. Beekman, K. W. et al. University of Chicago Consortium phase II study of ispinesib (SB-715992) in patients (pts) with advanced renal cell carcinoma (RCC) [abstract]. J. Clin. Oncol. 25 (18 Suppl.), a15573 (2007). 32. Gomez, H. L. et al. A phase I/II trial of ispinesib, a kinesin spindle protein (KSP) inhibitor, dosed q14d in patients with advanced breast cancer previously untreated with chemotherapy for metastatic disease or recurrence [abstract]. J. Clin. Oncol. 27 (15 Suppl.) a1077 (2009). 33. O'Connor, O. A. et al. A phase I-II trial of the kinesin spindle protein (KSP) inhibitor SB-743921 on days 1 and 15 every 28 days in non-Hodgkin or Hodgkin lymphoma [abstract]. J. Clin. Oncol. 26 (Suppl.), a8539 (2008). 34. Gerecitano, J. F. et al. A phase I/II trial of the kinesin spindle protein (KSP) inhibitor SB-743921 dosed q14d without and with prophylactic G-CSF in non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma (HL) [abstract]. J. Clin. Oncol. 27 (15 Suppl.) a8578 (2009). 35. Stein, M. N. et al. Phase I clinical and pharmacokinetic (PK) trial of the kinesin spindle protein (KSP) inhibitor MK-0731 in patients with solid tumors [abstract]. J. Clin. Oncol. 25 (18 Suppl.), a2548 (2007). 36. Goncalves, P. H. et al. A phase I study of ARRY-520 in solid tumors [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a2570 (2010). 37. Shah, J. J. et al. Phase I trial of ARRY-520 in relapsed/refractory multiple myeloma (RR MM) [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a8132 (2010). 2









