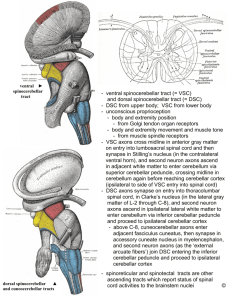
Anterior turning of commissural axons after midline crossing guided
advertisement

Anterior turning of commissural axons after midline crossing guided by Wnt/Frizzled signalling. Anna I. Lyuksyutova2, Chin-Chun Lu1, Nancy Milanesio1, Leslie A. King2 , Nini Guo4, Yanshu wang4, Jeremy Nathans4, Marc Tessier-Lavigne5 and Yimin Zou1,2,3 1 Department of Neurobiology, Pharmacology and Physiology, 2Committee on Developmental Biology and 3Committee on Neurobiology; 4Departments of Molecular Biology and Ophthalmology, HHMI, Johns Hopkins Medical School; 5Department of Biological Sciences, HHMI, Stanford University The molecular mechanisms that guide axons along anterior-posterior axis are unknown in both vertebrates and invertebrates. Commissural neurons in the alar plate of the spinal cord send axons towards the floor plate at the ventral midline, where they cross to the contralateral side and turn sharply anteriorly to project to the brain. Using explant assays, we have obtained evidence that chemoattractant gradient inside the spinal cord controls this guidance along the anterior-posterior axis. Several Wnt proteins expressed in the spinal cord can stimulate the extension of post-crossing but not pre-crossing commissural axons. Wnt4 and Wnt7b are expressed in the floor plate, where the anterior turning decision is made, and Wnt4 is expressed in a decreasing anterior-posterior gradient in the floor plate. sFRPs, inhibitors of Wnts, can disrupt the anterior-posterior pathfinding of post-crossing axons, and a localized source of Wnt4 protein can rescue the anterior turn of the misrouting axons. Finally, commissural axons in fz3 knockout mice display anterior-posterior guidance defects after midline crossing. Our results identify that Wnt proteins are candidate cues for guiding or polarizing axons along the anteriorposterior axis, connecting the spinal cord and the brain. Genome-wide association mapping in a founder population identifies ITGB3 as a QTL for whole blood serotonin levels Lauren Weiss, Jeremy Veenstra-VanderWeele, Suzanne Cheng, Mark Abney, Edwin Cook Jr. and Carole Ober Departments of Human Genetics and Psychiatry Serotonin has vital roles in the cardiovascular, intestinal, pulmonary and nervous systems. The few ancestral genomes and extensive LD present in the Hutterites, as well as a homogeneous environment resulting from their communal lifestyle, make the Hutterites an ideal population in which to map quantitative trait loci (QTLs). We conducted genome-wide association and linkage mapping studies to identify QTLs for serotonin levels in the Hutterites. The most significant association was a SNP (Leu33Pro) in integrin 3 (ITGB3) at 66 cM on chr 17 (P-value = 9.75 x 10-5). By homozygosity by descent (HBD) linkage mapping, a modest peak on chr 17 at 70 cM was also observed (LOD = 1.87, P-value = 0.0033). When genotype for ITGB3 Leu33Pro was included as a covariate in the linkage analysis, the linkage peak was nearly completely eliminated (LOD = 0.02, P-value = 0.76 at 67 cM). This indicates that either this SNP or a polymorphism in strong LD with it is responsible for the linkage signal in this region. In fact, further studies of variation in this gene have identified 6 SNPs spanning the gene that show more significant association with serotonin levels, indicating that additional variation may be relevant. The association of serotonin levels with a novel candidate, ITGB3, and the fact that it accounts for nearly all the evidence of linkage in the region suggest that this gene may be an important serotonin QTL.