Preservation versus Division of Ilioinguinal Nerve
advertisement
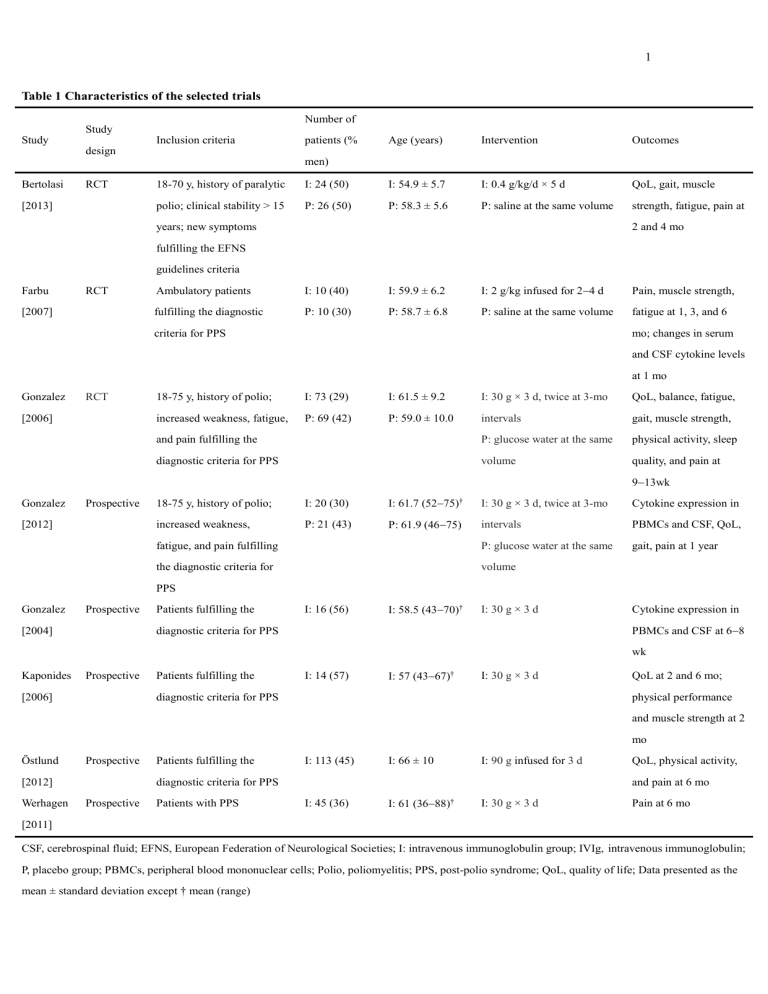
1 Table 1 Characteristics of the selected trials Number of Study Study Inclusion criteria patients (% Age (years) Intervention Outcomes design men) Bertolasi RCT [2013] 18-70 y, history of paralytic I: 24 (50) I: 54.9 ± 5.7 I: 0.4 g/kg/d × 5 d QoL, gait, muscle polio; clinical stability > 15 P: 26 (50) P: 58.3 ± 5.6 P: saline at the same volume strength, fatigue, pain at years; new symptoms 2 and 4 mo fulfilling the EFNS guidelines criteria Farbu RCT [2007] Ambulatory patients I: 10 (40) I: 59.9 ± 6.2 I: 2 g/kg infused for 24 d Pain, muscle strength, fulfilling the diagnostic P: 10 (30) P: 58.7 ± 6.8 P: saline at the same volume fatigue at 1, 3, and 6 criteria for PPS mo; changes in serum and CSF cytokine levels at 1 mo Gonzalez RCT [2006] 18-75 y, history of polio; I: 73 (29) I: 61.5 ± 9.2 I: 30 g × 3 d, twice at 3-mo QoL, balance, fatigue, increased weakness, fatigue, P: 69 (42) P: 59.0 ± 10.0 intervals gait, muscle strength, and pain fulfilling the P: glucose water at the same physical activity, sleep diagnostic criteria for PPS volume quality, and pain at 913wk Gonzalez Prospective [2012] 18-75 y, history of polio; I: 20 (30) I: 61.7 (5275)† I: 30 g × 3 d, twice at 3-mo Cytokine expression in increased weakness, P: 21 (43) P: 61.9 (4675) intervals PBMCs and CSF, QoL, fatigue, and pain fulfilling P: glucose water at the same gait, pain at 1 year the diagnostic criteria for volume PPS Gonzalez Prospective [2004] Patients fulfilling the I: 16 (56) I: 58.5 (4370)† I: 30 g × 3 d diagnostic criteria for PPS Cytokine expression in PBMCs and CSF at 68 wk Kaponides Prospective [2006] Patients fulfilling the I: 14 (57) I: 57 (4367)† I: 30 g × 3 d diagnostic criteria for PPS QoL at 2 and 6 mo; physical performance and muscle strength at 2 mo Östlund Prospective [2012] Werhagen Patients fulfilling the I: 113 (45) I: 66 ± 10 I: 90 g infused for 3 d diagnostic criteria for PPS Prospective Patients with PPS QoL, physical activity, and pain at 6 mo I: 45 (36) I: 61 (3688)† I: 30 g × 3 d Pain at 6 mo [2011] CSF, cerebrospinal fluid; EFNS, European Federation of Neurological Societies; I: intravenous immunoglobulin group; IVIg, intravenous immunoglobulin; P, placebo group; PBMCs, peripheral blood mononuclear cells; Polio, poliomyelitis; PPS, post-polio syndrome; QoL, quality of life; Data presented as the mean ± standard deviation except † mean (range) 2
![013—BD Global [DOC 117KB]](http://s3.studylib.net/store/data/005892885_1-a45a410358e3d741161b3db5a319267b-300x300.png)










