Raman spectroscopy for measurement of blood analytes
advertisement
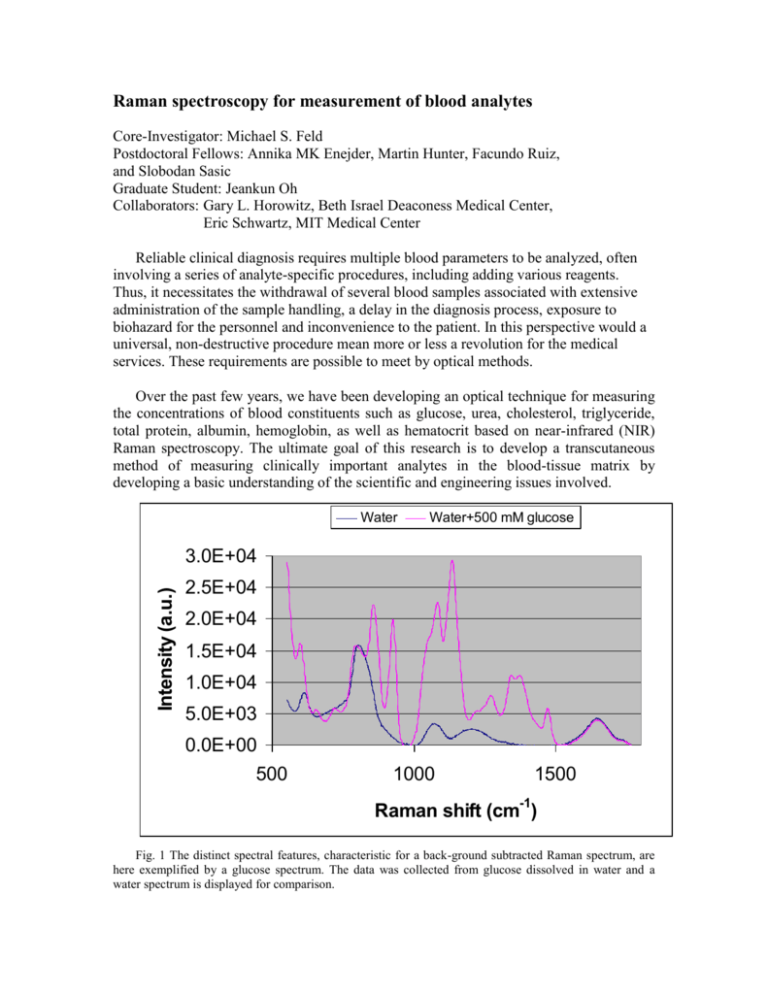
Raman spectroscopy for measurement of blood analytes Core-Investigator: Michael S. Feld Postdoctoral Fellows: Annika MK Enejder, Martin Hunter, Facundo Ruiz, and Slobodan Sasic Graduate Student: Jeankun Oh Collaborators: Gary L. Horowitz, Beth Israel Deaconess Medical Center, Eric Schwartz, MIT Medical Center Reliable clinical diagnosis requires multiple blood parameters to be analyzed, often involving a series of analyte-specific procedures, including adding various reagents. Thus, it necessitates the withdrawal of several blood samples associated with extensive administration of the sample handling, a delay in the diagnosis process, exposure to biohazard for the personnel and inconvenience to the patient. In this perspective would a universal, non-destructive procedure mean more or less a revolution for the medical services. These requirements are possible to meet by optical methods. Over the past few years, we have been developing an optical technique for measuring the concentrations of blood constituents such as glucose, urea, cholesterol, triglyceride, total protein, albumin, hemoglobin, as well as hematocrit based on near-infrared (NIR) Raman spectroscopy. The ultimate goal of this research is to develop a transcutaneous method of measuring clinically important analytes in the blood-tissue matrix by developing a basic understanding of the scientific and engineering issues involved. Water Water+500 mM glucose Intensity (a.u.) 3.0E+04 2.5E+04 2.0E+04 1.5E+04 1.0E+04 5.0E+03 0.0E+00 500 1000 1500 Raman shift (cm-1) Fig. 1 The distinct spectral features, characteristic for a back-ground subtracted Raman spectrum, are here exemplified by a glucose spectrum. The data was collected from glucose dissolved in water and a water spectrum is displayed for comparison. Raman spectra are composed of sharp and distinct features, characteristic for each molecule. Thus, one analyte can easily be distinguished from another, as well as from a broad fluorescence background. A typical Raman spectrum of glucose, being one of the blood constituents presently receiving much attention due to its importance in the treatment of diabetes patients, is shown in Fig. 1. However, to measure glucose in tissue is far more complex than indicated by this figure for various reasons: a) typical physiological concentrations are in the order of hundred times lower than that of the water solution in Fig. 1, b) the contribution of the fluorescence background from the surrounding tissue dominates the signal, c) Raman peaks from all biomolecules present may overlap and therefore interfere and d) the optical properties of the tissue influence the signal as well as the probe depth/volume. An attempt to visualize the challenging task of measuring glucose in whole blood is made in Fig. 2. whole blood glucose x 1000 glucose at physiological concentration (100 mg/dL) Fig. 2 A Raman spectrum collected from whole blood, containing a physiological amount of glucose, is shown in blue. It consists of a dominating fluorescence background on top of which the Raman peaks from all present biomolecules is superimposed. For comparison, the component originating from glucose is shown in red, as well as in black being magnified with a factor of 1000. We meet this stimulating task by optimizing the sensitive Raman instrumentation for the turbid character of tissue. In addition, we apply and develop powerful multivariate analysis techniques in order to extract the subtle signals generated by the analytes. In an initial multi-patient study [Koo et al 1999, Berger et al 1999] clinically relevant prediction accuracies were obtained for analytes in serum, and recently also in whole blood. Recent Publications “Measurement of blood analytes in turbid biological tissue using near-infrared Raman spectroscopy”, Tae-Woong Koo, Doctoral thesis, Massachusetts Institute of Technology, 2001 “Prospects for In Vivo Raman Spectroscopy", Eugene B. Hanlon, Ramasamy Manoharan, Tae-Woong Koo, Karen E. Shafer, Jason T. Motz, Maryann Fitzmaurice, John R. Kramer, Irving Itzkan, Ramachandra R. Dasari, and Michael S. Feld, Physics in Medicine and Biology, Volume 45, Number 2, pp. R1-R59, February, 2000. "Reagentless Blood Analysis by Near-Infrared Raman Spectroscopy", Tae-Woong Koo, Andrew J. Berger, Irving Itzkan, Gary Horowitz, and Michael S. Feld, Diabetes Technology & Therapeutics, Volume 1, Number 2, pp. 153-157, June, 1999 "Multicomponent Blood Analysis by Near-Infrared Raman Spectroscopy", Andrew J. Berger, Tae-Woong Koo, Irving Itzkan, Gary Horowitz, and Michael S. Feld, Applied Optics, Volume 38, Number 13, pp. 2916-2926, May, 1999 "Measurement of Glucose in Human Blood Serum using Raman Spectroscopy", TaeWoong Koo, Andrew J. Berger, Irving Itzkan, Gary Horowitz, and Michael S. Feld, IEEE-LEOS Newsletter, Volume 12, Number 2, p. 18, April, 1998 "An Enhanced Algorithm for Linear Multivariate Calibration", Andrew J. Berger, TaeWoong Koo, Irving Itzkan, and Michael S. Feld, Analytical Chemistry, Volume 70, Number 3, pp. 623-627, February, 1998 Recent Conference Presentation “Measurement of Blood Analytes in Human Whole Blood using Raman Spectroscopy”, Tae-Woong Koo, Andrew J. Berger, Irving Itzkan, Gary L. Horowitz, and Michael S. Feld, EAS, Atlantic City, NJ, November, 2000 “Reagentless Blood Analysis by Near-Infrared Raman Spectroscopy with Hybrid Linear Analysis”, Tae-Woong Koo, Andrew J. Berger, Irving Itzkan, Gary L. Horowitz, and Michael S. Feld, FACSS, Vancouver, Canada, October, 1999 Support This research is funded by National Center for Research Resources (National Institute of Health) and Bayer Diagnostics.