The Pharmacy Vocational Training Scheme

Template for Medicines Information January 2013
The Pharmacy Vocational Training Scheme: Stage 2
Template for evidence for competency Medicines information
Section1
Trainee name Personal portfolio reference
Title of enquiry
Category of enquiry Note requirement is a range of enquiries n= 10.Plac
e a ‘x’ in the box to indicate the category of enquiry
Administration/Dosage
Adverse effects
Availability/Supply
Identification
Interaction
Pharmaceutical
Pregnancy Breastfeeding
Choice of therapy/indications/contraindications
Compatibility of injections
Complementary medicine
Other, please specify ___________________________________
Complexity grade of enquiry (see Appendix 1)
Level 1 Level 2 Level 3
Medicines Information Centre Reference enquiry number (where applicable)
This is a confirmation that this enquiry being submitted for evidence demonstrates the following competencies
I23 I25 I26 I27 I29 I21 I22
Additional comments
Specialist pharmacist name
Specialist pharmacist signature
Date
Section 2
1
Template for Medicines Information January 2013
Question
Pertinent background information
Route of receipt of enquiry
Telephone In person
Urgent enquiry? Yes/ No
Summary of answer given to enquirer
E-mail Letter Fax
2
Template for Medicines Information January 2013
Route of delivery of summary answer
Telephone In person E-mail
Answered within the requested timescale Yes /No
Section 3
Letter Fax
Feedback received from enquirer on satisfaction with answer (where applicable)
3
Template for Medicines Information January 2013
Section 4
Reflections from undertaking enquiry
What went well? Is there anything you would you do differently in future? How will you apply the learning in your practice? What are the benefits of undertaking this learning to your practice.
4
Template for Medicines Information January 2013
Appendix 1 – Definition for enquiry levels
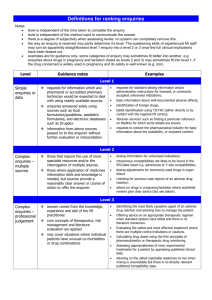
Level 1
Simple enquiries – answered using data from one or two standard sources.
[MiDatabank label: “Simple enquiries or data”]
Level 1 enquiries are generally requests for information which any pharmacist or accredited pharmacy technician would be expected to deal with using readily available sources. These can be answered using authoritative general reference texts e.g. BNF, SmPC, Martindale. However, enquiries answered solely using sources such as local formularies/guidelines, paediatric formularies, and electronic databases such as Drugdex would also be considered level 1.
For level 1 enquiries, the information found in the above sources can be passed on to the enquirer without further evaluation or interpretation. Many types of enquiry fit into this category.
Common examples include:
requests for standard dosing information and/or administration instructions for licensed, or commonly accepted unlicensed indications;
basic information about well-documented adverse effects;
identification of foreign drugs,
tablet identification using TICTAC (either directly or by contact with the regional MI centre);
‘librarian services’ such as finding a particular reference on Medline for which some details are known;
requests to contact the pharmaceutical industry for basic information about the availability, or excipient content.
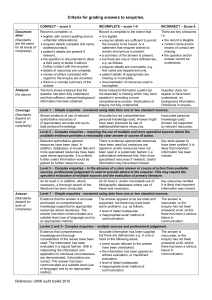
Level 2
Complex enquiries – requiring the use of multiple and more specialist sources where the available evidence provides a reasonably clear answer or course of action.
[MiDatabank label: “Complex enquiries – multiple sources”]
Level 2 enquiries require the use of more specialist resources and/or the interrogation of multiple sources. The application of medicines information skills and knowledge is needed, but sources provide a reasonably clear answer or course of action to offer the enquirer.
This is a broad category of enquiries comprising those that either require use of information sources in addition to those outlined under level 1, or require some evaluation and interpretation to be applied to a specific situation. Examples include:
dosing information for unlicensed indications;
intravenous compatibilities not likely to be found in the SPC/data sheet e.g. admixtures or Ysite compatibilities;
dosing adjustments for commonly-used drugs in organ failure
checking for previous case reports of an adverse drug reaction;
advice on drugs in pregnancy/lactation where published reviews give clear advice (but see below).
5
Template for Medicines Information January 2013
Level 3
Complex enquiries – in the absence of a clear answer or course of action from available sources, professional judgment is used to provide advice to the enquirer. This may require the specialist evaluation of multiple sources and the evaluation of primary literature.
[MiDataba nk label: “Complex enquiries – professional judgment”]
The emphasis in this level of enquiry is on professional judgment and accountability. A major component of the answer comes from the knowledge, experience and skill of the MI practitioner.
This means that core concepts of therapeutics, risk management and literature evaluation are applied to construct an answer. Examples include:
Identifying the most likely causative agent of an adverse drug reaction and advising how to manage the patient.
Offering advice on an appropriate therapeutic regimen when standard options have failed and there is no literature consensus.
Evaluating the safest and most effective treatment where there are multiple contra-indications or cautions.
Calculating drug doses using the first principles of pharmacokinetics or therapeutic drug monitoring.
Assessing appropriateness of new/ experimental treatments for a patient by appraising published clinical data.
Advising on the safest injectable medicines to mix when mixing is unavoidable but there is no directly relevant published compatibility data.
Enquiries that are likely to fall into this category include those regarding comparative effectiveness or safety of treatments, and situations where individual patients have unusual comorbidities or drug combinations.
6