Is There a Place for Ovarian Surgery
advertisement

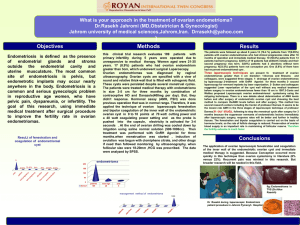
308866359 The Enigma of Polycystic Ovary Syndrome – Is There a Place for Ovarian Surgery? S. Daya Departments of Obstetrics and Gynecology and Clinical Epidemiology & Biostatistics, McMaster University, Hamilton, Ontario, Canada Polycystic ovary syndrome (PCOS) is a relatively common clinical disorder that affects 5 – 10 % of women in the reproductive age group. The disorder is associated with a wide spectrum of presenting features including anovulation, infertility, obesity, hirsutism, insulin resistance and dyslipoproteinemia. A fairly consistent finding on ultrasonography is enlargement of the ovaries with more than 10 sonolucent cystic structures, 2 – 8 mm in diameter scattered around an echo-dense thickened central stroma (1). Problems in inducing ovulation in PCOS are well recognized and range from a brisk response to no response at all. Obesity, even of moderate degree (BMI>27kg/m²) is associated with a reduced likelihood of ovulation. Thus, exercise and calorie restriction to reduce weight are necessary to optimize the success rate with medical therapy. There is also increased interest in the use of insulin sensitizing drugs to induce ovulation. However, it is not clear whether the observed benefit can be attributed to the medication or to the resulting weight loss. The drug of choice to induce ovulation is clomiphene citrate. It is an antiestrogenic agent that displaces endogenous estrogen from hypothalamic and pituitary estrogen receptor sites thereby reducing the negative feedback, resulting in an increased pulstile secretion of gonadotropin-releasing hormone (GnRH). The resulting rise in follicle stimulating hormone (FSH) that induces follicular growth is also accompanied by an equally large increase in serum luteinizing hormone (LH) concentration that may persist into the latter stages of the follicular phase of the cycle. Hypersecretion of LH is association with a lower likelihood of pregnancy and higher rate of miscarriage. 1 308866359 Nevertheless, high rates of ovulation (60 – 85%) and acceptable rates of pregnancy (30 – 40%) are seen with clomiphene citrate use, with most pregnancies occurring within 6 cycles of treatment. The reason for the failure to ovulate in the remaining 15 – 40% of anovulatory women who do not respond to clomiphene citrate is not clear. Gonadotropin Therapy Failure to ovulate after 2-3 successive cycles of clomiphene citrate at the maximal dose is usually an indication to consider treatment with gonadotropins. Advances in purification techniques have witnessed a progression in gonadotropin availability from menotropins to urofollitropins to the currently available follitropins. Women who respond normally to clomiphene citrate but fail to conceive after 6 to 12 cycles of treatment are also considered candidates for gonadotropin therapy. A variety of treatment regimens is available, but the low-dose, step-up regimen appears to produce optimal results with high cumulative pregnancy rates (62% after 6 months and 73% after 12 months of treatment) (2). The major risks associated with gonadotropin therapy are ovarian hyperstimulation syndrome (OHSS) and multiple pregnancy, both of which can be reduced with more careful monitoring with transvaginal ultrasonography and measurement of estradiol levels. Surgical Therapy Bilateral ovarian edge resection was first introduced as treatment for patients with anovulatory PCOS (3). This operation restored menstruation in all cases, and 2 out of 7 women became pregnant. Until the introduction of clomiphene citrate in 1962, this surgical approach was the only treatment available for women with anovulatory PCOS. The ovulation rates were high but pregnancy rates were much lower, probably because 2 308866359 of the high incidence of post-surgical periadnexal adhesion formation which converted an endocrinological problem to a mechanical one. Laparoscopic Ovarian Drilling This technique was first described in 1984 and involved the creation of 8 – 15 holes, each one 2 – 4mm deep on the surface and stroma of each ovary using a unipolar electrode at 300 – 400 W for 2 – 4 seconds (4). Several modifications of the technique have been reported including the use of laser (CO2, argon, KTP or Nd-YAG) with good results. However, the published results of laser surgery are inferior to those of electrocautery. Ovulation rates of 70 – 92% and pregnancy of rate of 70% have been reported. The use of an insulated needle for cautery was recently reported to be associated with minimal amount of adhesion formation in the small sample of patients who underwent a second-look laparoscopy (5). The technique involves inserting an insulated needle perpendicularly to the ovarian surface, entry being aided by a short duration of cutting current of 100 W. The whole length of the needle (8mm) is inserted into the ovary and activated with 40 W coagulating current for 2 seconds at each point. After making 10 – 15 punctures per ovary, the surface of the ovary is lavaged and 500 1000 ml of crystalloid solution is left in the peritoneal cavity. Mechanism of Action of Laparoscopic Ovarian Drilling The mechanism of action is unknown but is believed to be related to endocrine changes that result from the procedure. A significant reduction in androgen (testosterone and androstenedione) levels in the serum has been observed (6,7). A transient increase in LH in the first 24 to 48 hours after surgery is followed by a decrease in serum LH concentration (8). The decrease is mainly in pulse amplitude rather than pulse 3 308866359 frequency (9) suggesting an endocrine effect at the level of the pituitary gland rather than the hypothalamus. First ovulation after surgery occurs randomly involving either ovary even in patients with unilateral ovarian cauterization (10). The size of the ovary does not affect the response to the ovarian cauterization; similar decreases in LH, FSH, DHEAS and testosterone levels were observed in women with normal (ovarian volume < 8cm³ or cross-sectional area < 10cm²) or enlarged ovaries. (11) These observations strongly indicate that the effect of surgery is mediated via a central mechanism rather than a direct effect on the treated ovary. Interestingly, glucose utilization and plasma insulin concentrations during an euglycemic-hyperinsulinemic clamp did not change after laparoscopic ovarian cauterization (7). In addition, no significant changes were observed for cholesterol, triglycerides and apolipoprotein concentrations (7). Potential Risks and Complications of Laparoscopic Ovarian Drilling Intra-operative complications from the surgical procedure are rare and include avulsion of utero-ovarian ligament from excessive traction and bleeding from the holes drilled in the ovary (12). Other risks are associated with the laparoscopy procedure itself and are increased with obesity, a condition commonly encountered in women with PCOS. Periadnexal adhesions are problematic because of their higher incidence. Unfortunately, the risk of adhesion formation has not been ascertained accurately because there is no standardized follow-up protocol that involves second-look laparoscopy for all patients at a specified time after the initial surgical procedure. Based on case series reports, the incidence of adhesion formation has ranged from 0 – 100%, with the risk being higher when laser is used compared to electrocautery (2). It has been suggested that adhesion 4 308866359 formation can be reduced by minimizing injury to the ovarian surface by restricting the number of punctures, using an insulated needle, activating the coagulating current only after the needle is inside the ovarian stoma and irrigating the peritoneal cavity at the end of the procedure (8). Another potential complication is premature ovarian failure. However, the theoretical possibility of this complication awaits long-term follow-up studies evaluating the effect of ovarian drilling on the age at which menopause occurs. Comparative Efficacy of Ovarian Drilling and Gonadotropin Stimulation The increasing interest in surgical treatment if PCOS has been fuelled by the widespread availability and use of operative laparoscopy. The rationale for the surgical approach is the same as when ovarian wedge resection was introduced i.e. reduction of ovarian androgen levels so that follicular development can take place by avoiding the atresia that results from excess androgens. However, the efficacy of this procedure for ovulation induction and pregnancy has to be compared against the standard therapy such as gonadotropins in women with PCOS resistant to clomiphene citrate. To date, there have been four randomized trials addressing this question (13 – 16). The studies all employed bilateral ovarian electrocautery and compared this intervention with three (15) to at least six cycles (13, 14, 16) of gonadotropin stimulation. In 3 of the 4 trials, the sample sizes were small, ranging from 29 to 56, whereas in the largest trial (16) the sample size was 168. High rates of ovulation were observed in all trials. Regarding pregnancy rates, there was significant heterogeneity of treatment effect, primarily from one study in which there was a non-significant beneficial effect with ovarian cauterization (13). The other three studies all showed benefit in favour of gonadotropin 5 308866359 stimulation, the effect being statistically significant only in one trial (16). Overall, the common odds ratio using the random effects model was 0.66 (95% confidence interval, (CI) 0.16 – 2.75).(figure 1) Although the overall pregnancy rates were similar in the two groups in the largest trial (68% became pregnant after 6 cycles with gonadotropins and 74% became pregnant after 12 cycles in the ovarian drilling group), the surgically treated group was deliberately contaminated by ovulation induction with clomiphene citrate in some women and recombinant FSH in the rest who did not conceive (16). When comparing the two groups without contamination, it is clear that the pregnancy rate is much higher on a per cycle basis with gonadotropins; after three cycles, the cumulative pregnancy rate was almost twice as high with gonadotropins and continued to rise, whereas, it levelled off in the surgical treatment arm (17), At 6 months, the cumulative ongoing pregnancy rate was 37% in the ovarian cautery group and 68% with gonadotropins (relative risk 0.54, 95% CI 0.39 – 0.76) (17). The rate of spontaneous abortion was not different in the two groups (OR = 0.54, 95% CI 0.20-1.47).(figure 1) No multiple pregnancies were observed among patients treated with ovarian drilling compared with gonadotropin stimulation (OR=0.16, 95% CI 0.04-0.67). (figure 1) Long Term GnRH Analogue Versus Ovarian Drilling The belief that elevation of LH is implicated in the pathophysiology of PCOS led to the idea that normalizing the LH concentration could have a positive effect on this disorder (18) Recently, a prospective study was undertaken to compare the efficacy of medical treatment with GnRH analogue and surgical treatment using ovarian drilling in women with PCOS, unresponsive to clomiphene citrate (19). The protocol in the medical group 6 308866359 involved down-regulation with GnRH analogue for 6 months together with add-back therapy with an oral contraceptive. The surgical group underwent laser drilling of both ovaries with a CO2 laser. Each group then underwent treatment for up to three cycles with a low-dose, step-up stimulation regimen with recombinant FSH. Both groups had similar hormonal profiles and ovulation occurred with similar frequencies. Although the sample size was small, the pregnancy rates per patient were similar in the two groups. Thus, long term treatment with GnRH analogues appears to be as successful as laparoscopic laser cauterization. Summary In women with clomiphene resistant PCOS, the options of using gonadotropins or ovarian drilling with electrocautery both result in ovulatory cycles and pregnancy. However, the surgical intervention is not as efficacious as ovulation induction with gonadotropins, unless the two interventions are used sequentially. Hormonal profiles after ovarian electrocautery demonstrate a reduction in the levels of LH, testosterone, DHEAS and androstenedione, but glucose utilization did not change indicating that insulin sensitivity is not improved. Insulin resistance is believed to be a major factor in the persistence of anovulation in women with PCOS. The inability of ovarian cauterization to affect insulin sensitivity may, in part, explain the lower efficacy of this approach. Another reason may be the higher likelihood of developing periadnexal adhesions following surgery, and adverse effect that may be reduced with less traumatic techniques. Thus, based on the evidence available to date, the value of ovarian surgery as a treatment of women with clomiphene citrate resistant PCOS is 7 308866359 questionable and should only be considered as a last resort after all other options, including gonadotropin stimulation and GnRH analogue down-regulation, have failed References 1. Adams J, Polson DW, Adbulwahid N, Morris DV, Franks S, Mason HD, et al. Multifollicular ovaries: Clinical and endocrine features and response to pulsatile gonadotropin – releasing hormone. Lancet 1985; 2:1375-1378. 2. Balen AH, Braat DDM, West C. Cumulative conception and live birth rates after treatment of anovulatory infertility. An analysis of the safety and efficacy of ovulation induction in 200 patients. Hum Reprod 1994; 9:15631570. 3. Stein IF, Cohen MR. Surgical treatment of bilateral polycystic ovaries. Am J. Obstet Gynecol 1939; 38:465-473. 4. Gjonnaess H. Polycystic ovarian syndrome treated by ovarian electrocautery through the laparoscope. Fertil Steril 1984; 49:956-960. 5. Felemban A, Tan SL, Tulandi T. Laparoscopic treatment of polycystic ovaries with insulated needle cautery: a reappraisal. Fertil Steril 2000; 73:266-269 6. Greenblatt EM, Casper RF. Endocrine changes after laparoscopic ovarian cautery in polycystic ovarian syndrome. Ann J Obstet Gynecol 1987; 16:279-285. 7. Lemieux S, Lewis GF, Ben-Chetrit A, Steiner G, Greenblatt EM. Correction of hyperandrogenemia by laparoscopic ovarian cautery in women with polycystic ovarian syndrome is not accompanied by improved 8 308866359 insulin sensitivity or lipid – lipoprotein levels. J Clin Endocrinnol Metab 1999; 84:4278-4282. 8. Sathanandan SM, Jacobs HS. Ovulation induction in patients with polycystic ovaries. In : Sathanandan SM, Jacobs HS (eds) Practical Guide to Ovulation Induction. Imperial College Press, London, 2002 pp 105-137. 9. Rossmanith WE, Keckstein J. Spatzier K, Lauritzen C. The impact of ovarian laser surgery on the gonadotropin secretion in women with polycystic ovarian disease. Clin Endocrinol 1991; 34:223-230. 10. Balen A, Jacobs HS. A prospective study comparing unilateral and bilateral laparoscopic ovarian diathermy in women with the polycystic ovary syndrome. Fertil Steril 1994; 62:921-925. 11. Alborzi S, Khodaee R, Parsanejad ME. Ovarian size and response to laparoscopic ovarian electro-cauterization in polycystic ovarian disease. Int J Obstet Gynecol 2001; 74:269-274. 12. Gurgan T, Yarali H, Urman B. Laparoscopic treatment of polycystic ovarian disease. Hum Reprod 1994; 9:573-577. 13. Lazovic G, Milacic D, Terzic M, Spremovic S, Mitijasevic S. Medicaments or surgical therapy of PCOS. Fertil Steril 1978; 70:472 (abstract). 14. Vegetti W, Ragni G, Baroni E, Testa G, Marsico S, Riccaboni A, Crosignani PG. Laparoscopic ovarian drilling versus low-dose pure FSH in anovulatory clomiphene – resistant patients with polycystic ovarian syndrome: a randomized prospective study. Hum Reprod 1998; 13:120 (abstract). 9 308866359 15. Farquhar CM, Williamson K, Gudex G, Johnson N, Garland J, Sadler L. A randomized trial of laparoscopic ovarian diathermy versus gonadotropin therapy for women with clomiphene resistant polycystic ovarian syndrome. The Cochrane Library 2002 Issue 2. 16. Bayram N, Van Wely M, Bossuyt P, Van der Veen F. Randomized clinical trial of laparoscopic electrocoagulation of the ovaries versus recombinant FSH for ovulation induction in subfertility associated with polycystic ovary syndrome. Abstract 0-148 of the 17th Annual meeting of the ESHRE, Lausanne, Switzerland, July 2001. 17. Daya S. Laparoscopic ovarian electrocautery in the treatment of clomiphene- resistant polycystic ovary syndrome. Evidence-based Obstet Gynecol 2001; 3:164-165. 18. Goni M, Markussis V, Tolis G. Efficacy of chronic therapy with the gonadotropin releasing hormone agonist decapeptyl in patients with polycystic ovary syndrome. Hum Reprod 1994; 9:1048-1052. 19. Muenstermann U, Kleinstein J. Long-term GnRH analogue treatment is equivalent to laparoscopic laser diathermy in polycystic ovarian syndrome patients with severe ovarian dysfunction. Hum Reprod 2000; 15:25262530. Figure 1. Outcomes from comparative evaluation of laparoscopic ovarian cauterization and gonadotropin ovulation induction. 10