to the file.
advertisement

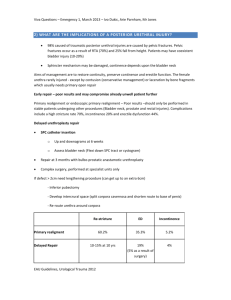
Muscle Invasive Bladder Cancer Marc C. Smaldone Fellow in Urologic Oncology, Fox Chase Cancer Center for Introduction It is estimated that bladder cancer will account for 14,680 disease specific deaths in 2010 in the United States, and represents the ninth leading cause of cancer-related death in men1. Epidemiologic studies do not suggest a hereditary cause of most bladder cancers2, although risk factors such as age, cigarette smoking, aromatic amines, aniline dyes, arsenic, and pelvic radiation have been described3. Although microscopic or gross painless hematuria is the presenting complaint in the vast majority of patients, irritative voiding symptoms such dysuria, urgency, and frequency are more common in patients with high grade papillary tumors, CIS, and invasive disease. The discovery of incidental bladder cancer at autopsies is very low, indicating that a vast majority of patients experience symptoms prompting workup or treatment during their lifetime4. Approximately 20-30% of patients present with muscle invasive disease, and of patients with non-muscle invasive tumors, 10-30% will progress to muscle invasion5. Clinical Staging The AUA Best Practice Policy on Asymptomatic Microscopic Hematuria defines microscopic hematuria as ≥3 red blood cells per high-power microscopic field from 2 of 3 properly collected urinalysis specimens6. All patients undergoing a hematuria evaluation should be evaluated with urine cytology, upper tract imaging, and cystoscopy5. In a patient with a bladder mass diagnosed on imaging or cystoscopy, an adequate transurethral resection is mandatory for histologic diagnosis and assessment for invasion. During the initial evaluation, the bladder, urethra, and upper tracts should be evaluated for presence of urothelial carcinoma. Tumors should be completely resected, and muscularis propria should be included to ensure accurate staging. Biopsy of the prostatic urethra should be performed in cases of bladder neck or prostatic urethral tumors, and random bladder mapping to rule out CIS can be performed when bladder sparing protocols are being considered or in the setting of a positive cytology with no obvious bladder or upper tract lesions4. In patients with bulky multifocal tumors, high grade Ta or T1 disease, or lack of detrusor muscle in the specimen, re-TUR resection is recommended as many as 20% of patients will be upstaged to muscle invasive disease following re-resection7. At the time of TUR, a bimanual exam under anesthesia should be performed to assess for local invasion. If a fixed or three dimensional mass is palpated, a clinical stage of ≥T3b is assigned8. Cross sectional imaging is an important component of the clinical staging workup when muscle invasive disease is present, and consists of imaging of the chest with chest radiography or computed tomography (CT) to evaluate for pulmonary metastases as well as abdominal imaging with CT or magnetic resonance imaging (MRI) to evaluate for local invasion, regional metastases, and systemic dissemination of disease. Although CT and MRI are limited in their ability to detect early metastatic disease, newer imaging modalities such as positron emission tomography (PET) have had poor results in patients with urothelial carcinoma due to urinary excretion and inconsistent tracer uptake9. Regardless of imaging modality, a thickened bladder wall or hydronephrosis raises suspicion for extravesical disease. Metastatic spread to the bone or brain is uncommon and additional imaging is not routinely indicated unless the patient has specific finding to indicate involvement. Despite significant improvement in imaging techniques, 30-50% of patients are upstaged at the time of cystectomy10. Pathologic Staging and Cancer Specific Outcomes Per the updated TNM staging system, organ confined disease invading the superficial or deep muscularis propria represents pT2a and pT2b disease respectively, while tumors that extend into the perivesical fat represent pT3 disease in a microscopic (pT3a) or macroscopic (pT3b) fashion. Tumors invading adjacent organs such as the prostatic stroma, seminal vesicles, uterus, or vagina, is classified as pT4a disease, and tumors invading the pelvic sidewall or abdominal wall meeting criteria for pT4b disease. Regional lymph node staging is determined by either single (N1) or multiple regional involved lymph nodes (N2), while metastases to the common iliac artery lymph node chain represents N3 disease3. Any nodal or distant metastatic disease is considered stage IV disease which imparts a very poor prognosis. The TNM system provides a useful system for stratifying prognosis, recurrence risk, and survival outcomes. With surgery alone, stratified by pathologic stage, 5 year survival rates are 66% for pT2 disease, 35% for pT3 disease, and 27% for pT4 disease respectively11. Treatment Options The gold standard treatment for clinically localized muscle invasive urothelial carcinoma is radical cystoprostatectomy or anterior pelvic exenteration with extended regional lymphadenectomy and creation of an incontinent or continent urinary diversion. Currently performed via an open or minimally invasive approach, large institutional series from centers of excellence report overall 5 year survival rates ranging from 45-66% with perioperative mortality rates less than or equal to 3%12, 13, and perioperative complication rates ranging from 25-57%13, 14 Recent evidence has suggested a 5-8% overall survival benefit with the administration of systemic chemotherapy15, 16, and current consensus recommends that neoadjuvant cisplatin based chemotherapy be considered in all patients with muscle-invasive urothelial carcinoma8. Poor candidates for upfront chemotherapy include patients with a poor performance status (ECOG ≥2) or poor renal function (eGFR <45-60)17. Although adjuvant therapy offers the advantage of being administered in only patients with known prognostic factors of recurrence its role has been poorly defined to date due to limited trials with methodological flaws. However, a recent meta-analysis of 6 randomized trials investigating the use of cisplatin based adjuvant therapy suggested a 25% relative reduction in the risk of death for patients receiving adjuvant therapy versus local therapy alone18. Less conventional treatments for muscle invasive bladder cancer include partial cystectomy, localized radiotherapy, and radical TUR in combination with radiotherapy and chemotherapy, which offer the potential for decreased morbidity, nerve preservation for potency, improved patient body image, and maintenance of bladder function. Using strict selection criteria including solitary primary tumors and absence of CIS, small series utilizing partial cystectomy have reported equivalent early oncologic results to radical cystectomy, although these findings are limited by selection bias 19. Historically, radiation therapy was reserved for patients unfit medically for radical cystectomy, or as palliative therapy in patients with locally advanced or metastatic disease20. Contemporary bladder-preserving protocols utilizing chemoradiation following complete TUR in patients with clinically staged muscle-invasive bladder cancer have reported complete response rates of 60-85%, 5-year survival rates of 50-60%, and survival rates with an intact bladder of 40-45%21. Important considerations for bladderpreserving therapy include careful patient selection; combined therapy with maximum TUR, radiation and concurrent chemotherapy; cystoscopic assessment of the response to therapy with prompt salvage cystectomy for non-responders or invasive recurrence. Although there are no randomized comparison studies with radical cystectomy, longterm contemporary data suggests that overall and disease-specific survival rates in contemporary bladder sparing protocols may be comparable to large radical cystectomy series in select patients22. However, until level I evidence exists, radical cystectomy will remain the treatment of choice in patients with localized muscle invasive urothelial carcinoma. In patients with locally advanced or systemic disease, the role of surgery shifts to palliation for the relief of symptoms including pain, bleeding, lower urinary tract symptoms, and fistula formation17. In patients with unresectable locally advanced primary tumors, palliative cystectomy or simply urinary diversion leaving the bladder in situ is a potential alternative in intractably symptomatic patients. In patients presenting with metastatic disease, cisplatin based chemotherapeutic regimens including methotrexate. vinblastine, doxorubicin, and cisplatin (MVAC) and gemcitabine/cisplatin (GC) have demonstrated comparable survival rates and response rates ranging as high as 46-49%23. Although these combinations show similar efficacy, GC is more tolerable with a significantly less toxic side effect profile. Carboplatin based therapy is less effective with less pronounced response rates and remains second line therapy24. Surveillance Although significant variation exists between protocols, patients with muscle invasive urothelial carcinoma are at high risk for recurrence/metastasis following cystectomy and require close surveillance. Per the NCCN guidelines, following cystectomy a urine cytology, serum chemistries, chest radiography, and abdominal/pelvic cross sectional imaging should be obtained every 3-12 months for two years and as clinically indicated8. A urethral washing is recommended every 6-12 months but recent data suggests that while uncommon (5-15%), urethral recurrences usually present symptomatically, and routine urethral washings do not appear to have a survival benefit25. Upper tract recurrences are also uncommon (<10%) and often tumors are not detected prior to the development of clinical symptoms. The most common distal sites for recurrence include lung, liver, and bone, and as many as 50% of patients will develop systemic disease following cystectomy26. Scoring algorithms based on independent predictors of recurrence at the time of cystectomy have been utilized to tailor surveillance protocols based on an individual patient’s pathologic characteristics and we expect the role of such algorithms to grow in the near future27. For bladder sparing protocols, patients should be evaluated with cystosopy, urine cytology, and consideration of bladder biopsy every 3-6 months for two years and then at increasing intervals3. References 1. Jemal, A., Siegel, R., Xu, J. et al.: Cancer statistics, 2010. CA Cancer J Clin, 60: 277, 2010 2. Golijanin, D. J., Kakiashvili, D., Madeb, R. R. et al.: Chemoprevention of bladder cancer. World J Urol, 24: 445, 2006 3. Jacobs, B. L., Lee, C. T., Montie, J. E.: Bladder cancer in 2010: how far have we come? CA Cancer J Clin, 60: 244, 2010 4. Sexton, W. J., Wiegand, L. R., Correa, J. J. et al.: Bladder cancer: a review of non-muscle invasive disease. Cancer Control, 17: 256, 2010 5. Hall, M. C., Chang, S. S., Dalbagni, G. et al.: Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol, 178: 2314, 2007 6. Grossfeld, G. D., Wolf, J. S., Jr., Litwan, M. S. et al.: Asymptomatic microscopic hematuria in adults: summary of the AUA best practice policy recommendations. Am Fam Physician, 63: 1145, 2001 7. Dalbagni, G., Vora, K., Kaag, M. et al.: Clinical outcome in a contemporary series of restaged patients with clinical T1 bladder cancer. Eur Urol, 56: 903, 2009 8. Montie, J. E., Clark, P. E., Eisenberger, M. A. et al.: Bladder cancer. J Natl Compr Canc Netw, 7: 8, 2009 9. Bouchelouche, K., Oehr, P.: Positron emission tomography and positron emission tomography/computerized tomography of urological malignancies: an update review. J Urol, 179: 34, 2008 10. Dutta, S. C., Smith, J. A., Jr., Shappell, S. B. et al.: Clinical under staging of high risk nonmuscle invasive urothelial carcinoma treated with radical cystectomy. J Urol, 166: 490, 2001 11. Herr, H. W., Dotan, Z., Donat, S. M. et al.: Defining optimal therapy for muscle invasive bladder cancer. J Urol, 177: 437, 2007 12. Dalbagni, G., Genega, E., Hashibe, M. et al.: Cystectomy for bladder cancer: a contemporary series. J Urol, 165: 1111, 2001 13. Stein, J. P., Lieskovsky, G., Cote, R. et al.: Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol, 19: 666, 2001 14. Svatek, R. S., Fisher, M. B., Matin, S. F. et al.: Risk factor analysis in a contemporary cystectomy cohort using standardized reporting methodology and adverse event criteria. J Urol, 183: 929, 2010 15. Grossman, H. B., Natale, R. B., Tangen, C. M. et al.: Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med, 349: 859, 2003 16. Winquist, E., Kirchner, T. S., Segal, R. et al.: Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and metaanalysis. J Urol, 171: 561, 2004 17. Stenzl, A., Cowan, N. C., De Santis, M. et al.: The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol, 55: 815, 2009 18. Adjuvant chemotherapy for invasive bladder cancer (individual patient data). Cochrane Database Syst Rev: CD006018, 2006 19. Smaldone, M. C., Jacobs, B. L., Smaldone, A. M. et al.: Long-term results of selective partial cystectomy for invasive urothelial bladder carcinoma. Urology, 72: 613, 2008 20. Coen, J. J., Zietman, A. L., Kaufman, D. S. et al.: Benchmarks achieved in the delivery of radiation therapy for muscle-invasive bladder cancer. Urol Oncol, 25: 76, 2007 21. Danesi, D. T., Arcangeli, G., Cruciani, E. et al.: Conservative treatment of invasive bladder carcinoma by transurethral resection, protracted intravenous infusion chemotherapy, and hyperfractionated radiotherapy: long term results. Cancer, 101: 2540, 2004 22. Mak, R. H., Zietman, A. L., Heney, N. M. et al.: Bladder preservation: optimizing radiotherapy and integrated treatment strategies. BJU Int, 102: 1345, 2008 23. von der Maase, H., Sengelov, L., Roberts, J. T. et al.: Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol, 23: 4602, 2005 24. Hussain, M., Vaishampayan, U., Du, W. et al.: Combination paclitaxel, carboplatin, and gemcitabine is an active treatment for advanced urothelial cancer. J Clin Oncol, 19: 2527, 2001 25. Lin, D. W., Herr, H. W., Dalbagni, G.: Value of urethral wash cytology in the retained male urethra after radical cystoprostatectomy. J Urol, 169: 961, 2003 26. Bochner, B. H., Montie, J. E., Lee, C. T.: Follow-up strategies and management of recurrence in urologic oncology bladder cancer: invasive bladder cancer. Urol Clin North Am, 30: 777, 2003 27. Umbreit, E. C., Crispen, P. L., Shimko, M. S. et al.: Multifactorial, sitespecific recurrence model after radical cystectomy for urothelial carcinoma. Cancer, 116: 3399, 2010 Marc C. Smaldone, MD Fellow in Urologic Oncology, Fox Chase Cancer Center