Clinical Chemistry Evaluations in Toxicity Studies
advertisement

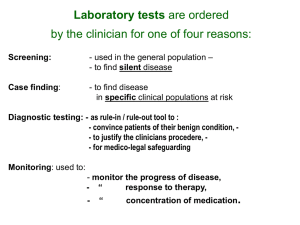
1 TOXICOLOGY 707 Clinical Chemistry Dr. Greg Travlos Fall 2007 Introduction Clinical chemistry evaluations are commonly recommended in animal toxicology studies. Some regulatory agencies (e.g., FDA, EPA) have set guidelines for clinical pathology testing in nonclinical toxicity and safety studies. Measurement of chemical components of biological fluids (e.g., serum, plasma, urine, CSF) afford the toxicologist some advantages compared to standard histopathology evaluations. Advantages include serial sampling, detection of metabolic injury, detection of organ specific effects, assistance in establishment of the no effect level and determination of toxic mechanism. There are practical problems that must be considered when including clinical chemistry evaluations in a study design. Choosing the appropriate endpoints for analysis, preanalytical sources of variation (e.g., age of animals used, diet, fasted v. non-fasted animals, site of blood collection, time of blood collection, sample handling/storage), blood volume required for testing (especially important when mice or very young rodents are used), species idiosyncrasies, processing large numbers of samples in a timely manner, appropriate instrumentation and methods, and establishment reference values (for the age, sex, and strain of laboratory animal) are examples of considerations that must be addressed by the laboratory investigator. Because clinical pathology testing of mice is limited by blood volume, interim, or in-life, sampling should not be performed and blood samples from mice should be collected at terminal sacrifice. Additionally, it often is not possible to perform both hematology and clinical chemistry analyses with the blood volumes obtained from mice. Therefore, if interim sampling times, or, both hematology and clinical chemistry analyses are required, additional mice should be added to the study protocol. Much emphasis has been placed on the use of serum enzymes as markers of tissue or organ damage. Enzymes are a highly specialized class of proteins that catalyze biochemical reactions that otherwise would occur at a very low rate. They catalyze reactions by complexing with the substrates and lowering the energy of activation without altering the equilibrium constant. They are not consumed during the reaction process. They are usually reaction specific, but not substrate specific, and act within a specific cellular location (e.g., cell membrane, cytosol, mitochondria). For an enzyme to be valuable diagnostically, it must reasonably reflect pathological change in a specific tissue, organ, or group of organs and must be easily measured. Alterations in cell membrane integrity and enzyme synthesis, release, catabolism, inactivation, inhibition, and excretion have all been implicated as effectors of serum enzyme activity. Another significant factor affecting tissue release is the enzyme concentration within a particular tissue. Obviously a tissue with a greater concentration of enzyme will contribute more to the serum than tissues with little or no concentration. The magnitude of a serum enzyme activity increase is not always a reflection of the severity of damage or the tissue concentration (e.g., ALT and ALP). Differences related to species, age, and sex should also be considered. Similar considerations hold true for measurements of other variables. Additionally, some constituents (e.g., hormones) may have cyclic variations. In 1986, the National Toxicology Program (NTP) revised its procedures for clinical pathology (hematology and clinical chemistry) evaluations in 13-week toxicity studies. Objectives of this were to standardize the approach to clinical pathology testing, produce relevant information on chemicals selected for study, and generate an extensive data base for the Fischer 344 rat and B6C3F1 mouse. Frequently, little information exists concerning the toxicity of the compounds selected for study. So a "core" of clinical chemistry tests was established, permitting evaluation of several organ systems. The use of a core removes guesswork from study design and prevents inadvertent omission of important endpoints. However, the use of a core does not preclude the inclusion of other relevant clinical pathology tests in a study. 2 The clinical chemistry tests in the NTP core include: Alanine aminotransferase Sorbitol dehydrogenase Alkaline phosphatase Total bile acids Creatine kinase Urea nitrogen Creatinine Total protein Albumin The purpose of this review is to provide general information concerning individual biochemical components and their application in a toxicology setting. Applied properly, these evaluations provide evidence of general or target-organ treatment effects at various time points, help identify possible mechanisms of action, and assist with establishment of no effect levels and dose selection for chronic studies. The relevance of the data obtained will depend on the appropriateness of time points sampled and the endpoints evaluated. Laboratory evaluation of liver: Hepatic impairment is well recognized as a toxicological problem and chemicals can cause a variety of functional and/or structural injuries. There are several types of laboratory tests that can be used to evaluate hepatic integrity/function. Analytical methods include, serum activities of enzymes of hepatic importance, tests of hepatic metabolism, and hepatic clearance/function tests. It would be recommended that at least two appropriate estimators of hepatocellular (e.g., alanine aminotransferase, sorbitol dehydrogenase, or total bile acids) and hepatobilliary (e.g., alkaline phosphatase, 5'-nucleotidase, or total bile acids) health be performed when evaluating the liver. A). Serum enzymes of hepatocellular injury: 1. Alanine aminotransferase (ALT): Alanine aminotransferase is a cytosolic enzyme that catalyzes the reaction: L-alanine + a-ketoglutarate pyruvate + glutamate Activity of ALT is found normally in serum and CSF, but not urine. It is stable at room, refrigerated, and frozen temperatures. Depending on the species, ALT has a biological half-life of approximately 48-60 hours. Cyclosporin decreases serum and liver tissue ALT activity as a result of ALT inhibition due to a drug metabolite. If vitamin B6 is added to the assay, ALT activity will be partially restored. The greatest tissue activity in laboratory rodents, rabbits, primates, dogs, cats, and man is found in the liver. In these species increased serum activity is used to indicate hepatocellular injury (leakage). Additionally, certain drugs can cause increases in liver ALT without causing histopathological hepatocellular change. For example, in rats, glucocorticoids can induce increased liver ALT activity, in a dose-related fashion, by as much as 13-fold. ALT is also found in low concentrations in skeletal and cardiac muscle of several laboratory animal species, including rats. In some laboratory animal species, for example the laboratory pig, ALT is found in higher concentrations in cardic and skeletal muscle than it is in liver. Thus, it is possible that serum ALT activity may increase related to muscle injury. For example, dogs were treated with CCL4 (liver necrosis) and plasmocid-dihyroiodide (muscle necrosis). Treatment with CCL4 caused increases in serum activity of ALT, AST, and SDH. Treatment with plasmocid-dihyroiodide caused increases in serum activity of ALT and AST only. 3 2. Sorbitol dehydrogenase (SDH): This is a liver-specific cytosolic enzyme that catalyzes the reaction: D-sorbitol D-fructose The enzyme is found in appreciable amounts in hepatocytes and testes. Hepatocellular damage, however, appears to be the only cause for increased serum activity. It is a diagnostically useful enzyme in all species for hepatocellular injury. SDH has a short circulating half-life (<6 hours). Thus, SDH may be useful for demonstrating an acute hepatic injury but may not be useful for long term monitoring. Metal chelating anticoagulants, such as EDTA, decrease SDH activity . In some species, SDH is not very stable in stored serum. In laboratory rodents, however, SDH is stable at refigerated temperatures for more than 2 days. 3. Aspartate aminotransferase (AST): Aspartate aminotransferases catalyze the reaction: L-aspartate + 2-oxoglutarate oxaloacetate + glutamate There are two isoenzymes of AST, one is located in the cytosol and the other is mitochondrial. AST is present in significant amounts in a wide variety of soft tissues, thus, precluding its use as marker of organ-specific injury. In general, increases of serum AST activity would be used as an indicator of soft tissue damage. Serum AST activity has been used as an indicator of hepatocellular injury/leakage. But, because AST is not tissue specific, the utility of AST as a diagnostic aid for hepatocellular damage has given way to more organ-specific serum indicators (e.g., SDH, ALT). AST activity in serum is stable and serum samples can be held at least 3 days at room temperature, 7 days at refrigerated temperatures, 30 days frozen. Red blood cells contain significant amounts of AST and red cell leakage or hemolysis can elevate serum AST activity. 4. Lactate dehydrogenase (LDH): LDH is a cytosolic enzyme which catalyzes the reaction: pyruvate L-lactate LDH exists as a tetramer of 2 protimers (designated H and M). There are 5 isoenzymes LDH1 (LDH-H4); LDH2 (LDH-H3M1); LDH3 (LDH-H2M2); LDH4 (LDH-H1M3); LDH5 (LDH-M4). LDH is found in most tissues in varying amounts and increases of serum LDH activity would be used as an indicator of soft tissue damage; isoenzyme profiles may have some utility to help identify tissue-specific injury. Red blood cells contain approximately 150-fold greater LDH activity than in serum. For most species, the LDH isoenzyme profile of red cells is similar to that of cardiac muscle (predominantly LDH1 and LDH2). However, in the rat, the LDH isoenzyme profile of red blood cells is similar to that of skeletal muscle (predominantly LDH5). Thus, care must be taken when collecting and handling the specimen to avoid hemolysis. LDH4 and LDH5 are cold labile and freezing should be avoided. Oxalate anticoagulants inhibit LDH activity. B). Laboratory evaluation of cholestasis: 1. Alkaline phosphatase (ALP): Alkaline phosphatase is a group of nonspecific enzymes that hydrolyze phosphate esters including ATP at an alkaline pH. ALP is ubiquitous and is found in most cells of the body. Intestine and kidney have the highest amounts of ALP but tissue concentration is generally not related to serum activity in disease (e.g., liver). ALP is primarily a membrane enzyme and is usually found on brush borders of secretory/absorptive epithelium. It is also found in significant amounts in bone and placenta. There are isoenzymes of ALP related to the expression of different genes. In man and some nonhuman primates, there are three genes coding for ALP; one for placental ALP, one for intestinal ALP, and one for others (liver, bone, kidney). In other mammals, including rats, there are just two genes coding for ALP; one for intestinal and one for the others. Isoforms identified for various tissues (for example, liver versus bone) are 4 antigenically similar because they are products of the same gene. The isoforms result from a posttranslational modification of the molecule but are enzymatically similar. The isoenzymes and isoforms can be identified immunologically and by varying sensitivities to heat inactivation and inhibitors (e.g., levamisol, L-phenylalanine). In the normal rat, circulating ALP is primarily the intestinal and bone fractions. In man normal ALP activity arises from bone, liver, and intestine; in pregnant women placenta is a major contributor. In general, young animals have higher serum ALP activity than adult animals due to increased bone activity. In rats, conditions that cause a decreased food intake cause a decrease in serum ALP activity due decreased contribution of the intestinal isoenzyme. Serum ALP activity is used as marker of hepatic injury, specifically cholestasis (e.g., bile duct ligation). ALP activity can increase with active bone disease (e.g., bone neoplasia). However, increases related to bone disease are usually mild. The diagnostic value of ALP is affected by the variable composition of serum ALP and the effects of diet and age. 2. 5'-Nucleotidase (5-NT): 5'-Nucleotidase is a membrane enzyme which catalyzes the hydrolysis of nucleoside 5monophosphates. 5-NT is found in many body tissues with the highest activities found in kidney and intestinal mucosa. Diagnostic utility of 5-NT have focused on the serum increases in 5-NT activity associated with hepatobilliary disease (e.g., cholestasis). While most tissues contain 5NT, the specificity this enzyme exhibits appears to be related to the observation that a detergent or bile acids are required for enzyme solubilization and release from membranes. Thus, biliary disease (particularly involving cholestasis) would provide optimum conditions for enzyme solubilization and release. 3. Total bile acids: Bile acids are synthesized in the liver from cholesterol. In most species, synthesis of cholic and chenodeoxycholic acids (known as primary bile acids) predominate. In the pig, chenodeoxycholic acid is hydroxylated in the liver to form hyocholic acid. The primary bile acids are predominantly taurine (rat and dog) or glycine (rabbit) conjugated, and released into the small intestine via the bile. In the intestine, they play an important role in the digestion and absorption of lipids and lipid-soluble vitamins. The daily need is greater than hepatic synthesis and demands are met by a significant enterohepatic recirculation of bile acids. Enteric bacteria modify the primary bile acids to secondary bile acids. For example, cholic acid deoxycholic acid and chenodeoxycholic acid lithocholic acid. Lithocholic acid is relatively insoluble and not reabsorbed; deoxycholic acid is reabsorbed in the large intestine and returned to the liver where it is either rehydroxylated to cholic acid and secreted or secreted as conjugated deoxycholic acid. Bile acid concentrations are sensitive indicators of cholestasis. Serum total bile acid concentrations can also be affected by mechanisms other than cholestasis (e.g., altered enterohepatic circulation or impaired hepatic function). Non-cholestatic liver injury can elevate circulating bile acid concentrations. In contrast, serum ALP activity increases minimally in response to hepatocellular damage. It has been demonstrated that increases in total bile acid concentration were useful for detecting early development of chloroform and carbon tetrachloride induced liver injury in male rats; ALP activity was not affected by either hepatotoxicant. 4. Bilirubin, direct and total: Bilirubin is a product of heme metabolism from a variety hemoproteins. The destruction of hemoglobin, myoglobin, and heme-containing enzymes leads to heme. Heme is first degraded by heme oxygenase to biliverdin (green). Further degradation by biliverdin reductase converts biliverdin to bilirubin (yellow). Most heme catabolism occurs in the spleen and liver. Plasma bilirubin concentration is directly proportional to the rate of heme turnover and inversely proportional to the rate of hepatic clearance. In health, plasma bilirubin is primarily in the unconjugated form. It is water insoluble and requires albumin as a transport protein. Unconjugated bilirubin is cleared by hepatocytes, bound to an intracellular organic ion (carrier) and conjugated with glucuronic acid. The conjugated bilirubin is then excreted in the bile. Increases in serum bilirubin concentrations can occur with excessive heme turnover (e.g., hemolytic disease), decreased hepatocellular uptake and conjugation (loss of hepatic mass or functional defect), and cholestasis. With cholestasis the bilirubin increase is primarily the 5 conjugated (direct) form (>50% of the total circulating bilirubin). The hyperbilirubinemia of hemolytic disease or decreased uptake or conjugation results in excess (>50% of the total) unconjugated bilirubin. An indication of where the defect is occurring can be narrowed down by measuring total and conjugated bilirubin. The unconjugated bilirubin can be calculated by subtracting the conjugated from the total bilirubin. Bilirubin uptake is competitively inhibited by the dyes BSP or ICG, but not bile acids. C). Laboratory evaluation of hepatic clearance/function; exogenous dyes: Dye clearance tests can be used as a measure of hepatic function. Sulfobromophthalein (BSP) and indocyanin green (ICG) are two cholephilic dyes that are commonly used. BSP administered IV binds to albumin and is transported to the liver. Hepatocytes remove the bound dye from the circulation and conjugate it to glutathione by the action of glutathione transferase. The conjugated dye (and a portion of free dye) is excreted in the bile. An estimate of dye clearance is obtained by measuring the dye remaining in the circulation in accurately timed, postadministration blood samples. delayed dye clearance can be seen with hepatic necrosis, fibrosis, cholestasis, and decreased hepatic blood flow. Approximately 50% of the functional liver mass must be lost before delayed clearance (increased retention) occurs. Decreased albumin concentrations cause an increased clearance. Laboratory evaluation of kidney: The kidneys play an important role in the regulation of water, electrolyte, and acid-base balance, and the removal of certain metabolic wastes and toxins. The functional unit (nephron) of the kidney has two functionally distinct parts: the glomerulus and the tubule system. The glomerulus acts as a semipermeable diffusion membrane while the tubule system acts on the glomerular ultrafiltrate to maintain water and solute homeostasis in the animal. Quantitative and qualitative serum and/or urine analyses are used evaluate kidney function. For general evaluation of kidney function, both serum urea nitrogen and creatinine concentrations should be performed. If, however, the compound being tested is a suspected renal toxicant, the serum testing should be supplemented with measurements of urine constituents in a timed (for rats and mice, 16-24 hour) urine collection. The urine must be collected in a metabolism cage and the urine container maintained cool during the collection period. A). Serum indicators of renal injury: Urea nitrogen (UN) and Creatinine (Cre): Serum concentrations of UN and Cre are traditional screening tests for kidney function and increased serum concentrations can provide evidence of renal dysfunction (e.g., decreased glomerular filtration rate). Urea provides a mechanism for ammonia excretion. UN synthesis occurs in the liver and is excreted in the urine. Creatinine is a metabolic waste product of muscle metabolism. Muscle contains phosphocreatine which undergoes spontaneous cyclization with loss of inorganic phosphorus to form creatinine. Conversion of creatine to creatinine is a nonezymatic, irreversible process occurring at the rate of about 1.6-2% per day. Both UN and Cre are used as estimators of the glomerular filtration rate and approximately 75% of the nephrons have to be nonfunctional before changes in serum concentration of these variables occur. Levels of serum UN can be influenced by many extrarenal causes. High protein diets, dehydration, liver function, animal health and nutritional status are factors that can affect serum UN concentration. Serum creatinine is not as affected by extrarenal factors and has been considered more sensitive than serum UN for detecting nephropathy. 6 B). Urine indicators of renal injury: 1. Physical characteristics Color/turbidity: Urine is usually yellow-amber and is caused by a urochrome related to a product of heme degradation. Abnormal constituents can alter urine color (e.g., hemoglobin or RBC's, bilirubin, X-ray contrast media, new methylene blue, BSP). Turbidity varies among species and individuals; usually urine is clear. Volume: Urine starts out at approximately the same osmolality as plasma. Water reabsorption, independent of body needs, occurs in the proximal tubules (this is an osmotic process). The urine is still isoosmotic to plasma as it enters the loop of Henle. The urine osmolality increases in the descending loop of Henle; it is highly permeable to water but impermeable to solute. The ascending loop of Henle is solute permeable but water impermeable, and urine osmolality decreases as the urine progresses to the distal tubules and collecting ducts. Fluid entering the distal tubule is hypoosmotic to plasma. Water reabsorption in the distal tubules and collecting ducts is in excess of solute and is related to body needs. It is under the influence of antidiuretic hormone (ADH) and is the primary control of urine volume. Osmolality/specific gravity: Estimates the solute concentration in the urine and is a good indicator of the concentrating ability of the kidney. Osmolality is measured by freezing point depression or vapor pressure while specific gravity is measured by hydrometry or refractometry. Osmolality is the method of choice for toxicology studies. 2. Chemical constituents Urine is a complex mixture containing most all the constituents found in plasma. However, due to the homeostatic role of the kidney, the concentrations of various urine constituents are markedly different from their plasma concentrations. Urine concentrations are affected by kidney function, animal hydration and nutritional status, and metabolic processes. Additionally, diurnal effects, even in a normal animal requires that urinary constituents be reported on a timed or per mg creatinine basis. Glucose and total urine protein are commonly measured and are used as indicators of proximal tubule function. Analyses of urine enzyme activity have been used as markers of renal tubule injury. Many enzymes have been evaluated, in the urine, as potential markers of renal injury. Of those studied, LDH, AST, ALP, ALT, N-acetyl-b-Dglucosaminidase (NAG), b-glucuronidase (b-GLU), acid phosphatase (ACP), and leucine aminopeptidase (LAP), have been considered useful indicators of renal injury. Because most of the enzymes evaluated in urine are large (>70,000 d) protein molecules and do not pass the glomerulus, the serum activity of the enzyme being measured usually has little bearing on the urinary activity. Practical considerations for the evaluation of enzymuria should include the size (i.e., molecular weight) of the enzymes of interest, the stability of the enzyme in urine, the direct (inhibitory/potentiating) effect of the test compound (parent/metabolite) on the enzyme, and the quality of the urine specimen collected (for example, in rats, it has been shown that fecal contamination can markedly increase the urinary activity of ALP and AST). Laboratory evaluation of muscle : 1. Creatine kinase (CK): CK catalyzes the reversible phosphorylation of creatine phosphate, the major storage form of high-energy required by muscle. creatine + ATP creatine phosphate + ADP There are three major isoenzymes of CK. They are dimers consisting of either muscle (M) or brain (B) subunits and are numbered CK1 (CK-BB), CK2 (CK-MB), and CK3 (CK-MM). CK is found in many cell types but has the highest specific activity in skeletal muscle. In the rabbit, dog, and horse CK3 is the dominant form in cardiac and skeletal muscle. Additionally, though the brain has high CK activity (CK1), very little if any activity is found in normal CSF or serum. Increases in CSF CK activity have been seen with neurological disease. Total serum CK 7 activity often increases in neurological disease, but is usually derived from muscle as a result of convulsions or recumbancy. CK is unstable stored at room, refrigerator, and frozen temperatures due to rapid oxidation of -SH groups at the active site. Addition of thiol agents to the assay will reactivate the enzyme. 2. Aspartate aminotransferase (AST) and lactate dehydrogenase (LDH): As mentioned previously, AST and LDH are present in significant amounts in a wide variety of soft tissues, thus, precluding their use as markers of organ-specific injury. However, because skeletal and cardiac muscle contain high amounts of AST and LDH activity, serum AST and LDH activity have been used as indicators of muscle injury. In the rat, cardiac muscle has the highest activity (269 IU/g tissue)a of AST than what has been found in other tissues (for example, liver, 88 IU/g; skeletal muscle, 69 IU/g; kidney, 57 IU/g; intestine, 25 IU/g)a. AST does have a longer biological half-life (~18 hours) than creatine kinase (~5 hours) and, thus, may be useful for demonstrating and/or following an acute skeletal or cardiac myopathy over a longer period of time. For LDH, tissue activities in the rat are high in various tissues (for example, liver, 212 IU/g; skeletal muscle, 453 IU/g; cardiac muscle, 360 IU/g; kidney, 167 IU/g; intestine, 185 IU/g)a. Because LDH consists of H and M subunits that combine to form a tetramer, there are 5 isoenzymes (LDH1, H4; LDH2, H3M1; LDH3, H2M2; LDH4, H1M3; LDH5, M4). In the rat, LDH5 predominates in skeletal muscle and LDH1 and LDH2 predominate in cardiac muscle. Thus, isoenzyme profiles may have some utility to help identify tissue-specific injury. However, LDH5 also predominates in other rat tissues (for example, liver and red blood cells); LDH1, LDH2 and LDH3 predominate in brain. Because the LDH isoenzyme profile of red blood cells is similar to that of skeletal muscle, care must be taken when collecting and handling the specimen to avoid hemolysis. Additionally, there is a wide variation in the circulating half-lives for the different isoenzymes (40 hours, LDH1; 3 hours, LDH5) which could interfere with the interpretation of LDH isoenzyme profiles. In general, increases of serum activity of AST or LDH would be used as indicators of soft tissue damage. And the introduction of serum creatine kinase activity as a specific marker of muscle disease/injury, has diminished the utility of AST and LDH for such disorders. a From Boyd, J. W. (1983). The mechanisms relating to increases in plasma enzymes and isoenzymes in diseases of animals. Vet. Clin. Pathol., vol. 12, 2:9-24. 3. Troponin: Troponins I and T (TnI and TnT, respectively) are globular proteins involved with the regulation of muscle contraction. Cardiac and skeletal muscle-specific isoforms of TnI and TnT have been identified and monoclonal immunoassays for the cardiac troponins have been developed. Several investigators have described the utility of cardiac TnI and TnT evaluations in diagnosis of myocardial infarction, and both were as sensitive and more specific than CK-MB isoenzyme. TnI and TnT immunoassays developed for the diagnosis of cardiac injury in humans have been tested in the laboratory pig heart and skeletal muscle tissues and have been demonstrated to be useful. O’Brien et al. (1997) suggested that TnI and TnT assays would be candidate biomarkers of cardiac injury and they concluded that TnI was the most specific cardiac biomarker for mammals described to date. In a coronary occlusion and reperfusion pig model, serum CK and TnT were used to evaluate the cardioprotective ability of C1 esterase inhibitor (C1INH) treatment. In the vehicle controls, CK and TnT increased with reperfusion. CK activity peaked 10 minutes after reperfusion then declined to elevated, but not significantly different, levels than the C1-INH treated animals at 120 minutes. TnT was also elevated at 10 minutes then continued to increase, peaking at 120 minutes, and remained elevated at 180 minutes after reperfusion; the increases were significantly different than the C1-INH treated animals at 150 and 180 minutes. 8 Other clinically important analyses: 1. Total Protein and Albumin: Plasma and serum proteins are a heterogeneous group consisting of albumin and , , and -globulins. Albumin is produced by the liver and constitutes approximately 25-50% of the total serum protein. Albumin acts to maintain osmotic pressure and as a transport protein. and -Globulins are also produced by the liver and act primarily as carrier compounds. -Globulins are synthesized by lymphoreticular cells and function as antibodies. Plasma fibrinogen is a liver produced globulin that is responsible for clot formation and is not found in serum. Total plasma protein values will be slightly higher than serum values due to the fibrinogen content. Total protein and albumin measurements are readily available and the globulin fraction can be obtained by subtraction: Total protein (g/dL) - Albumin (g/dL) = Globulin (g/dL) Decreases in protein values can be caused by several factors including hyperhydration, albumin and/or protein loss in renal or intestinal disease, impaired protein synthesis (hepatic or lymphoreticular), increased catabolism, and poor nutritional status (e.g., malnutrition, malabsorption, pancreatic insufficiency). Increases in protein can be related to dehydration, inflammatory disease (acute or chronic), and neoplasia. Increases in albumin concentration occur as a result of dehydration and no other cause. 2. Glucose: Serum glucose concentration is utilized as an estimation of carbohydrate metabolism and has been extensively studied in both rodent and non-rodent animal models of diabetes mellitus. Serum glucose concentration is affected by a large number of preanalytical and analytical sources of variation. For example, differences in methods of animal handling, restraint, anesthesia, blood collection, fasting v. non-fasting, diet, post-collection sample processing, and method of glucose determination can cause variation of the serum concentrations obtained. In blood kept at room temperature, glucose concentrations will decrease approximately 10% per hour due to the continued utilization (i.e., glycolysis) of this nutrient by red blood cells; separate serum from the red cells as soon as possible. Blood glucose concentrations also can be protected from glycolysis by the addition of the anticoagulant sodium floride to the collection vial (10 mg/mL of blood). 3. Cholesterol and Triglycerides: Cholesterol, cholesteryl esters, phospholipids, and triglycerides are the primary constituents of plasma lipids. Plasma lipids are transported in the circulation as large macromolecules called lipoproteins; they consist of different ratios of various lipids (cholesterol, cholesteryl esters, phospholipids, etc.) and specific proteins (apolipoproteins). Lipoproteins include chylomicrons, very low-density lipoproteins (VLDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL). There are species variations regarding the proportion of each type of lipoprotein found in the plasma. For example, in humans, approximately two thirds of the plasma cholesterol is carried as LDL; in rats, a species that is relatively resistant to the induction of hyperlipidemia, approximately 60% of the plasma cholesterol is carried as HDL. Measurement of serum cholesterol and triglyceride concentrations are relatively simple to perform and accurate and are used as estimators of lipid metabolism; they have been extensively studied in animal models of atherosclerosis and hyperlipidemia. Factors, such as, diet (e.g., fat and cholesterol content) and fasting v. non-fasting can have a major impact on circulating concentrations of these lipid constituents. 4. Electrolytes: Electrolytes play a vital role in many biological processes. Electrolytes are responsible for the maintenance of body fluid pH, the maintenance of the dynamic balance of water volume between the two major body fluid compartments (specifically, the intracellular fluid, ICF, volume and extracellular fluid, ECF, volume), the creation of transmembrane electrical potentials that result nerve conduction and muscle contractions, and they severe as cofactors for many enzyme 9 mediated reactions. Electrolytes consist of both cations and anions; the primary electrolytes are listed below. Cations mEq/La Anions mEq/L sodium (Na+) potassium (K+) 4.3 calcium (Ca2+) 2.5 magnesium (Mg2+) 142.0 chloride (Cl-) bicarbonate (HCO3-) phosphates (PO4-,2-) proteins 14.0 others (sulfates, lactate, etc.) total anions 104.0 24.0 2.0 total cations 1.1 149.9 5.9 149.9 From Tasker, J. B. (1980). In “Clinical Biochemistry of Domestic Animals” (J. J. Kaneko, ed.), 3rd Ed., pp. 402-447. Academic Press, NY. a Serum is best for measurement of electrolyte concentrations and the serum should be separated from the clot as soon as possible to avoid concentration alteration related to red cell hemolysis or leakage in vitro. Analysis by flame photometry (for Na+ and K+) and coulombmetric titration (for Cl-) can result in erroneously low values if the samples are lipemic or hyperproteinemic; measurement of electrolytes by ion-specific electrodes are not affected by increases in the non-aqueous phase (e.g., lipemia). Besides evaluating alterations of the concentration of individual electrolytes, electrolyte concentrations may be used to estimate changes in acid-base balance (for example, anion gap = (Na+ + K+) - (Cl- + HCO3-); where the HCO3- is estimated by measuring total serum CO2) and to estimate the expected serum osmolality (for example, mOsm/kg = 2(Na+ + K+); when serum urea nitrogen or glucose concentrations are high then, mOsm/kg = 2(Na+ + K+) + glucose/18 + urea nitrogen/2.8; in both formulas, Na+ and K+ are measured in mEq/L and glucose and urea nitrogen are measured in mg/dL). Because their serum concentrations are linked too parathyroid function, calcium (Ca2+), phosphorus (PO4), and magnesium (Mg2+) are electrolytes that warrant special consideration. The parathyroid gland produces the hormone parathormone (PTH) in response to a decreased serum Ca2+ concentration (hypocalcemia) and, possibly, to a hypomagnesemia. Additionally, hyperphosphatemia causes a decrease in serum calcium and, thus, can stimulate increased PTH production indirectly. Most methods for calcium (measured as total serum calcium), PO4 (measured as inorganic phosphate), and Mg2+ are colorimetric and results are often reported in mg/dL. Erroneously increased Ca2+ values occur with lipemia; erroneously decreased Ca2+ values occur with hypoalbuminemia. Falsely increased PO4 values occur with hemolysis. Total serum Ca2+ is approximately 40-50% ionized (free form), 30-40% protein bound (primarily to albumin), and 10% complexed to other anions (e.g., phosphates). It is only the ionized form that is biologically active in bone and cellular metabolism, neuromuscular activity, and coagulation. Besides special analytical methods, it is essential that samples collected for ionized Ca2+ determination be fresh and held anaerobically at 37oC. Ionized Ca2+ concentration will change with alterations of pH. For example, any decrease in pH, or increase of temperature, in a sample will cause an increase in the proportion of ionized Ca2+. 5. Methemoglobin: The measurement of methemoglobin (MetHb) is used as an estimate of oxidative injury to red blood cells (RBC’s). MetHb is a hemoglobin (Hb) molecule of which the iron moiety of the heme units is in the ferric instead of the ferrous state. Oxidative hemolysis occurs when a compound overwhelms or impairs the antioxidant systems of RBC's. Red blood cells are protected from oxidant stress by a variety of mechanisms, including superoxide dismutase, catalase, reduced glutathione, methemoglobin reductase, and vitamins C and E. Oxidative damage can involve hemoglobin or the red cell membrane. Reticulocytes are more resistant to oxidative injury than mature RBC's because they have increased activity of the enzyme systems involved in oxidant reduction. 10 An example of chemical MetHb former is phenylhydroxylamine a. Phenylhydroxylamine (PHA) is a n-hydroxylamine that causes RBC oxidative stress oxidizing Hb to MetHb. Normally, DeoxyHb is in the Fe+2 state, while OxyHb is in the Fe+3 state; an e- is transferred to bound O2 giving bound superoxide ion (O2-). During deoxygenation, the e- returns to the iron moiety and O2 is released. Oxidation to MetHb occurs when O2- is replaced with Cl- during deoxygenation (<3%/day). Some MetHb and Heinz body formers produce oxidative damage by donating e- to OxyHb. Hb3+: O2- + PHA Hb3+:O22- + .PHA Hb3+: O22- + 2H+ Hb3+ + H2O2 The drug free radical generated (.PHA) can now donate the unpaired e- to molecular O2 to form superoxide or to another OxyHb to form more MetHb and H2O2. Free radicals achieve stability by extracting e- from SH groups of Hb, enzymes, membrane proteins, GSH, by oxidizing membrane unsaturated fatty acids, or by oxidizing NADH or NADPH. When the tritratible SH groups of Hb are oxidized beyond the protective process (GSH) a conformational change occurs exposing more SH groups for oxidation. The end result is denaturation and precipitation of Hb. Not all MetHb formers cause Heinz bodies (e.g., nitrites). Some produce both (e.g., chloronitrobenzenes, copper). a From Harvey, J. W. (1989). In “Clinical Biochemistry of Domestic Animals” (J. J. Kaneko, ed.), 4th Ed., pp. 185-234. Academic Press, NY. 6. Cholinesterase (ChE): Serum ChE activity is composed of two distinct cholinesterases; acetylcholinesterase (AChE) and butyrylcholinesterase (ButChE). Acetylcholine is the major substrate. Acetylcholine is neurotransmitter found at myoneural junctions and AChE found at the myoneural junction is essential in hydrolyzing acetylcholine so the junction can be reestablished. Myoneural AChE is also found in RBC's. In serum, ButChE is the primary type and AChE is minimal. Both AChE and ButChE have similar inhibitors and activators; inhibition of one reflects inhibition of the other. Currently the most important inhibitors of cholinesterases are the organophosphates and carbamates. Serum is the specimen of choice. Stability of ButChE is variable; lasting ~6 hr. at room temperature, weeks at refrigerator temperatures, and months at frozen temperatures.