Lung Cancer Patient Data Form
advertisement
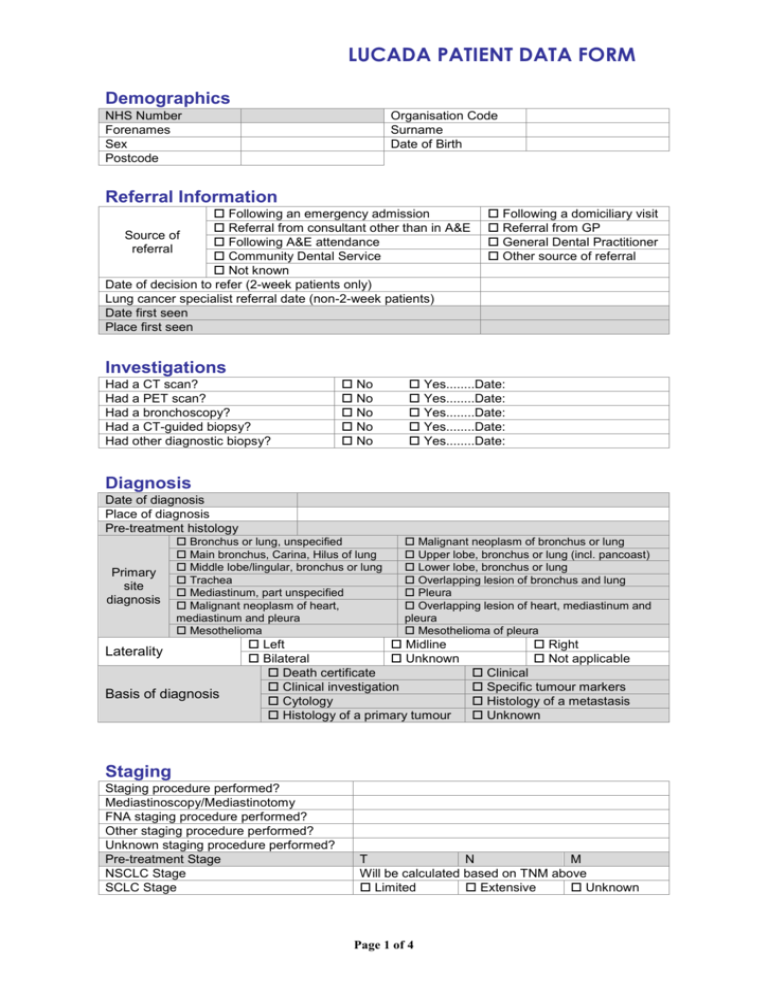
LUCADA PATIENT DATA FORM Demographics NHS Number Forenames Sex Postcode Organisation Code Surname Date of Birth Referral Information Following an emergency admission Referral from consultant other than in A&E Source of Following A&E attendance referral Community Dental Service Not known Date of decision to refer (2-week patients only) Lung cancer specialist referral date (non-2-week patients) Date first seen Place first seen Following a domiciliary visit Referral from GP General Dental Practitioner Other source of referral Investigations Had a CT scan? Had a PET scan? Had a bronchoscopy? Had a CT-guided biopsy? Had other diagnostic biopsy? No No No No No Yes........Date: Yes........Date: Yes........Date: Yes........Date: Yes........Date: Diagnosis Date of diagnosis Place of diagnosis Pre-treatment histology Primary site diagnosis Bronchus or lung, unspecified Main bronchus, Carina, Hilus of lung Middle lobe/lingular, bronchus or lung Trachea Mediastinum, part unspecified Malignant neoplasm of heart, mediastinum and pleura Mesothelioma Laterality Basis of diagnosis Malignant neoplasm of bronchus or lung Upper lobe, bronchus or lung (incl. pancoast) Lower lobe, bronchus or lung Overlapping lesion of bronchus and lung Pleura Overlapping lesion of heart, mediastinum and pleura Mesothelioma of pleura Left Midline Bilateral Unknown Death certificate Clinical investigation Cytology Histology of a primary tumour Right Not applicable Clinical Specific tumour markers Histology of a metastasis Unknown Staging Staging procedure performed? Mediastinoscopy/Mediastinotomy FNA staging procedure performed? Other staging procedure performed? Unknown staging procedure performed? Pre-treatment Stage NSCLC Stage SCLC Stage T N M Will be calculated based on TNM above Limited Extensive Unknown Page 1 of 4 LUCADA PATIENT DATA FORM Care Plan/MDT Discussed at MDT? Treatment intent Treatment modalities Suggested plan Yes…….Date: No Unknown Curative Palliative Palliative (supportive care only) Unknown No specific anti-cancer treatment Single modality Multiple modality Unknown Surgery Radiotherapy Chemotherapy Brachytherapy Palliative care Active monitoring Sequential chemotherapy and radiotherapy Concurrent chemotherapy and radiotherapy Induction chemo to downstage before surgery Neo-adjuvant chemotherapy and surgery Surgery followed by chemotherapy Co-Morbidities Was there any reason why the patient did not receive the first choice of treatment? Died COPD Refused Co-morbidity precluding treatment Dementia/Cerebrovascular disease Renal failure Severe weight loss Co-morbidities FEV1 Absolute FEV1 percentage Performance Status 0 1 2 3 Cardiovascular disease Other malignancy Other 4 Not recorded Treatment - Surgery Hospital code Date of decision to operate Date of surgery Main surgical procedure Completeness of resection Wedge resection of lesion of lung Multiple wedges resected Segmental resection Sleeve resection Lung resection with resection of chest wall (not identifying which lobe resection) Carinal resection Lobectomy Pneumonectomy Open operation on lung (open and close) Bilobectomy Other open operation on lung Extrapleural pneumonectomy Debulking pleurectomy Pleurodesis Presence of No residual Microscopic Macroscopic residual tumour tumour residual tumour residual tumour cannot be assessed Surgical histology Date of surgical histology Pathological stage Pathological NSCLC Stage Pathological SCLC Stage pT Page 2 of 4 pN pM LUCADA PATIENT DATA FORM Treatment - Chemotherapy Hospital code Date of decision to treat Date of start of treatment Chemotherapy intent Chemotherapy alone Neo-adjuvant chemotherapy before surgery Part of a chemotherapy / radiotherapy treatment plan Adjuvant chemotherapy post surgery Induction chemotherapy to down stage before surgery Treatment - Radiotherapy Hospital code Date of decision to treat Date of start of treatment Radiotherapy site Radiotherapy intent Trachea Lung Mediastinum Skin Chest wall Bone Mesothelioma drain site Other Region of Body Brain Curative (radical) radiotherapy Curative (CHART / CHARTWEL) Part of a chemotherapy / radiotherapy treatment plan Adjuvent following surgical treatment Palliative Radiotherapy Treatment - Brachytherapy Hospital code Date of decision to treat Date of start of treatment Treatment – Palliative Care Hospital code Date of decision to treat Date of start of treatment Palliative Care Provider Type Palliative Care Community Provider Palliative Care Intervention Given Hospital Community Hospice Nursing Home Home care Other Unknown No Yes........Date: Treatment – Active Monitoring Hospital code Date of decision to treat Page 3 of 4 LUCADA PATIENT DATA FORM Outcomes Trial status Date of death Was death treatment-related? Morbidity type Was PCI given Was the original plan carried out? Reason for failure of original plan Patient eligible, consented to and entered trial Patient not entered into clinical trial Clinical trial status unknown Yes No Unknown Surgery Chemotherapy Radiotherapy Combination Yes No Unknown Yes No Unknown Cancer progressed through treatment such that a new treatment plan required Patient choice Patient died Treatment toxicity Disease progression Lung Cancer Nurse Specialist Was patient assessed by LCNS? Date of first assessment by LCNS How was patient first assessed by LCNS? At what stage was the patient assessed by LCNS? Was LCNS present when patient received their diagnosis? Yes No In clinic Ward Visit Other Not recorded Before diagnosis Before and after diagnosis Unknown Yes No Notes Page 4 of 4 Unknown Home visit Telephone Unknown After diagnosis At diagnosis only Not recorded Unknown











