ONLINE APPENDIX SUPPLEMENTAL METHODS Data Quality
advertisement
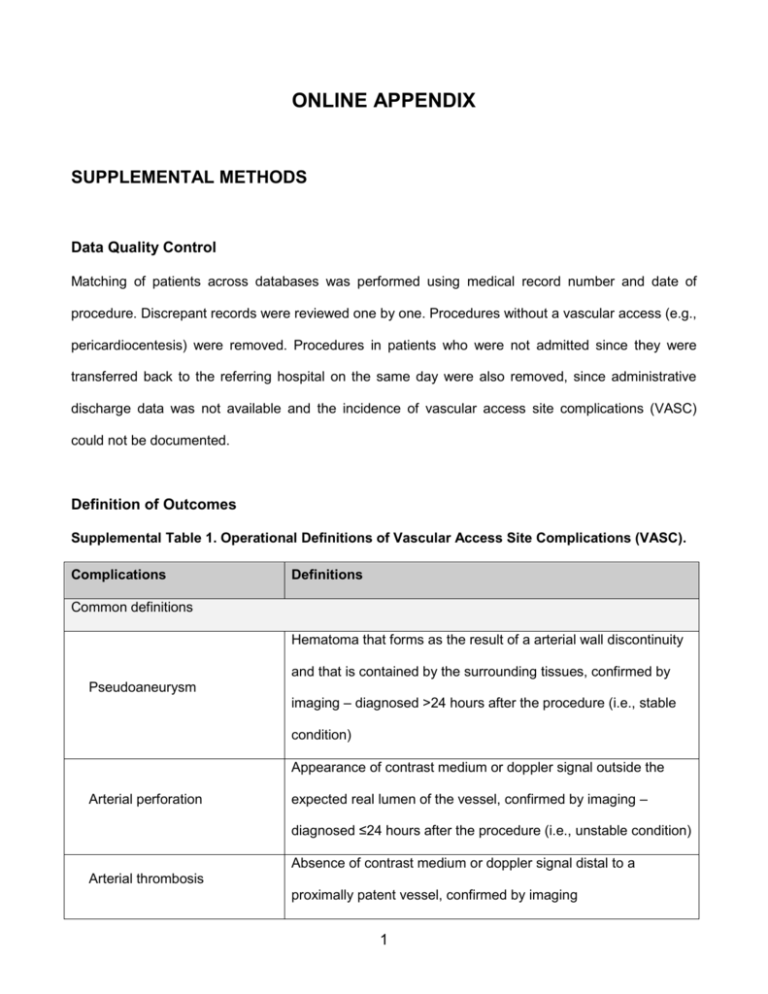
ONLINE APPENDIX SUPPLEMENTAL METHODS Data Quality Control Matching of patients across databases was performed using medical record number and date of procedure. Discrepant records were reviewed one by one. Procedures without a vascular access (e.g., pericardiocentesis) were removed. Procedures in patients who were not admitted since they were transferred back to the referring hospital on the same day were also removed, since administrative discharge data was not available and the incidence of vascular access site complications (VASC) could not be documented. Definition of Outcomes Supplemental Table 1. Operational Definitions of Vascular Access Site Complications (VASC). Complications Definitions Common definitions Hematoma that forms as the result of a arterial wall discontinuity and that is contained by the surrounding tissues, confirmed by Pseudoaneurysm imaging – diagnosed >24 hours after the procedure (i.e., stable condition) Appearance of contrast medium or doppler signal outside the Arterial perforation expected real lumen of the vessel, confirmed by imaging – diagnosed ≤24 hours after the procedure (i.e., unstable condition) Absence of contrast medium or doppler signal distal to a Arterial thrombosis proximally patent vessel, confirmed by imaging 1 Appearance of contrast medium or of a doppler signal outside the Arterial dissection expected vessel lumen with longitudinal extension (real-andfalse-lumen image), confirmed by imaging Local access site signs of infection, with or without Local infection microbiological or biochemical evidence, or fever Definition specific to radial access Major hematoma [1] Local induration ≥5 cm in the forearm, arm or shoulder Definitions specific to femoral access Local induration ≥10 cm measured at the puncture site [2]; ≥5 cm Major hematoma if a vascular closure device was used [1,3] Bleeding into the retroperitoneal space, either confirmed by Retroperitoneal hematoma imaging or with the combined occurrence lumbar/abdominal pain, hemodynamic instability and sudden drop in hemoglobin Communication between the femoral artery and vein, confirmed Arterio-venous fistula by imaging Ischemia distal to entry site with an entry site that is patent and Distal embolization with normal flow, confirmed by imaging Paresthesia, dysesthesia, hypoesthesia and/or pain that follows Nerve irritation nerve distribution Attributable Risk The primary objective of this study was to ascertain, at a population level, the fraction of VASC in femorally-accessed (FA) patients of the contemporary cohort that is attributable to the use of radial access (RA). To this end, we used the concept of attributable risk, as outlined in Figure 1A [4]. 2 Attributable risk can be interpreted as the proportion of disease cases over a specified time period that would be prevented following elimination of the exposure, assuming the exposure is causal [5]. Figure 1. Attributable Risk (AR). Operationally, to investigate the inverse relationship between RA safety benefits and FA safety hazards, attributable risks were calculated with the formula shown in Figure 1B. Patients from the historical cohort were considered as the unexposed group, while FA patients from the contemporary cohort were considered as the exposed group. Charlson-Deyo and Elixhauser Comorbidity Indexes For the Charlson-Deyo comorbidity index (CDCI), we used the scoring system proposed in the original paper by Charlson et al. [6]. Since a scoring system was not described in the original paper by Elixhauser et al. [7], for the Elixhauser comorbidity index (ECI) we attributed one point for the presence of each comorbidity. To account for the change in coding standards between the two study periods (1996-1998 and 2006-2008), a validated code conversion scheme was used to match the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9 CM) coding scheme 3 in use during the first period with the International Classification of Diseases, 10th Revision (ICD-10) coding scheme in use during the second period [8]. Supplemental Table 2. Diseases Included in the Charlson-Deyo and Elixhauser Comorbidity Indexes. Charlson-Deyo comorbidity index Elixhauser comorbidity index 1. Myocardial infarction (1) 1. Congestive heart failure 2. Congestive heart failure (1) 2. Cardiac arrhythmias 3. Peripheral vascular disease (1) 3. Valvular disease 4. Cerebrovascular disease (1) 4. Pulmonary circulation disorders 5. Dementia (1) 5. Peripheral vascular disorders 6. Chronic pulmonary disease (1) 6. Hypertension 7. Rheumatic disease (1) 7. Paralysis 8. Peptic ulcer disease (1) 8. Other neurological disorders 9. Mild liver disease (1) 9. Chronic pulmonary disease 10. Diabetes without chronic complication (1) 10. Diabetes, uncomplicated 11. Diabetes with chronic complication (2) 11. Diabetes, complicated 12. Hemiplegia or paraplegia (2) 12. Hypothyroidism 13. Renal disease (2) 13. Renal failure 14. Any malignancy, including lymphoma and 14. Liver disease leukemia, except malignant neoplasm of 15. Peptic ulcer disease excluding bleeding skin (2) 16. Acquired immune deficiency syndrome 15. Moderate or severe liver disease (3) 17. Lymphoma 16. Metastatic solid tumor (6) 18. Metastatic cancer 17. Acquired immune deficiency syndrome / 19. Solid tumor without metastasis Human immunodeficiency virus (6) 20. Rheumatoid arthritis/collagen vascular 4 diseases 21. Coagulopathy 22. Obesity 23. Weight loss 24. Fluid and electrolyte disorders 25. Blood loss anemia 26. Deficiency anemias 27. Alcohol abuse 28. Drug abuse 29. Psychoses 30. Depression Note: For the CDCI, the scoring system is detailed between parentheses; for liver disease, diabetes and malignancy, the most severe degree of disease is considered (if a solid tumor with metastasis is present, score only for metastatic tumor). For the Elixhauser comorbidity index, two points could be assigned to hypertension depending on the severity of the presentation. Predictors of Vascular Access Site Complications in the Femoral Contemporary Cohort To identify the predictors of VASC in the femoral contemporary cohort, we performed univariate logistic regressions followed by stepwise regression. A p-value <0.20 in univariate regression was required for a variable to enter the stepwise model and a p-value <0.05 was required to remain in the final multivariate model. The following variables were considered as candidate predictors: age, sex, body mass index, type of procedure, access site crossover, vascular closure device, anticoagulant, use of glycoprotein IIb/IIIa inhibitors, period of the year, ECI and sheath size. The results of these analyses are outlined in Online Table 2. 5 Stratified analyses We performed several analyses, stratifying VASC rates and attributable risks by: 1. ECI subcategories (0, 1, 2, ≥3): we used these fixed values of ECI when multiplying by the beta coefficients of the variables included in the model used to calculate adjusted VASC rates. We subsequently estimated the rates and attributable risks of VASC in the contemporary FA cohort as compared with the historical cohort, according to ECI subcategories (Figure 3). 2. VASC risk score: we used the beta coefficients of the independent predictors of a VASC among contemporary FA patients (Online Table 2B), to calculate the baseline risk of a VASC for subjects in the contemporary FA cohort and historical cohort. We subsequently performed a stratified analysis to determine the rates and attributable risks of VASC in the contemporary FA cohort as compared with the historical cohort, according to quartiles of VASC risk (Figure 4). 3. Propensity of undergoing FA in the contemporary cohort: we created a propensity score model to stratify subjects of the contemporary cohort with similar baseline and procedural characteristics. The propensity model included: age, gender, body mass index, diabetes, dyslipidemia, hypertension, smoking, prior history of coronary artery disease, peripheral artery disease, stroke/TIA, heart failure, CABG and chronic kidney disease, as well as the ECI, the CDCI, presentation with STEMI, cardiogenic shock, cardiac arrest, type of procedure (therapeutic vs. diagnostic) and sheath size. The propensity model allowed good balance since standardized differences between radial and femoral within each stratum were <0.10 in 80% of cases. We subsequently performed a stratified analysis to determine the rates and attributable risks of VASC among contemporary FA patients, according to quintiles of the propensity score of undergoing femoral access, and compared them to the overall VASC rate in the historical cohort (Figure 5). References 6 1. Beraldo de Andrade P, Piva e Mattos LA, Tebet MA, et al. Design and rationale of the AngioSeal versus the Radial approach In acute coronary SyndromE (ARISE) trial: a randomized comparison of a vascular closure device versus the radial approach to prevent vascular access site complications in non-ST-segment elev. Trials 2013;14:435. 2. Chandrasekar B, Doucet S, Bilodeau L, et al. Complications of cardiac catheterization in the current era: a single-center experience. Catheter. Cardiovasc. Interv. 2001;52:289–95. 3. Larsen EN, Hansen CB, Thayssen P, Jensen LO. Immediate mobilization after coronary angiography or percutaneous coronary intervention following hemostasis with the AngioSeal vascular closure device (the MOBS study). Eur. J. Cardiovasc. Nurs. 2013;:[Epub ahead of print]. 4. Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am. J. Public Health 1998;88:15–9. 5. Levine B. What does the population attributable fraction mean? Prev. Chronic. Dis. 2007;4:A14– A14. 6. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40:373–83. 7. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med. Care 1998;36:8–27. 8. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9CM and ICD-10 administrative data. Med. Care 2005;43:1130–9. 7







