RILEY ANESTHESIA PROCEDURE MANUAL
advertisement

RILEY ANESTHESIA PROCEDURE MANUAL
Purpose: This manual was compiled
procedures for anesthesia in Riley
gives you some feel for how to set
this way are left for your study.
study of pediatric anesthesia, and
to facilitate understanding of standard operating
Children's Hospital. It is an abbreviated outline that
up a case. The more important issues of why we do it
This manual does not substitute for your independent
is not a peer-reviewed document.
Former IU Residents: Former IUMC residents can get a free current copy of this Procedure
Manual by contacting Thomas Wolfe, MD Riley Hosp Rm 2001, 702 Barnhill Drive, Indpls
46202, phone 274-9981, e-mail thomas_m_wolfe@yahoo.com. A copy can be attached and sent
as a Word document, or a hard copy mailed to you.
Pediatric Anesthesia Drugs
ETT & LMA sizes
Fluid Guidelines
Introduction to the Riley OR
Phone numbers
Pain Management
Magnetic Resonance Imaging (MRI)
Radiation Therapy
Total Body Irradiations
Gastrointestinal Endoscopic Procedures &
Liver Biopsies
Selected ENT Procedures
Laser Excision of Papillomas
Bronchoscopy for Foreign Body
Palatoplasty
Pulse Dye Lasers (PDL’s)
Dental Restorations
Selected Ophthalmologic Procedures
Selected GU (Genitourinary) Procedures
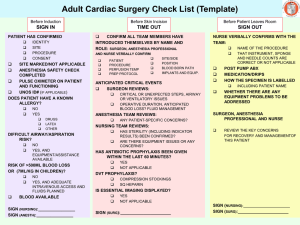
Open Heart Procedures
Patent Ductus Arteriosus
Coarctation of Aorta
Blalock-Taussig Shunt
Pulmonary Artery Banding
Cardiac Catheterization & EP Lab
SBE Prophylaxis
Abbreviations in congenital heart disease
Name:
Edition: July 2007
Spinal Fusions
Evoked Potential Reference Guide
Venticuloperitoneal Shunts (VP Shunts)
Myelomeningocele Closure
Craniectomy
Mediastinal Masses
Pectus Excavatum Repair
Herniorraphy
Nissen Fundoplication
Intra-abdominal tumors (neuroblastoma &
Wilm's)
Neonatal Surgery (anoplasty, TEF,
diaphragmatic hernia, gastroschisis,
omphalocele, NEC, pyloric stenosis,
congenital lobar emphysema)
Cardiac Transplant
Liver Transplant
Renal Transplant
Bone Marrow Transplant / Harvesting
Juvenile (Type I) Diabetics
Sickle Cell Disease
Muscular dystrophies
Pediatric Burns
Latex Allergy
Malignant Hyperthermia
Pediatric Resuscitation
STANDARD DRUGS IN PEDIATRIC ANESTHESIA
Inhaled Anesth
MAC (child)
MAC (adult)
Bld/Gas Sol
Brain/Bld Sol
Vapor Press
7.5%
2.5%
1.8%
1.1%
---
6.0%
2.0%
1.15%
0.75%
105%
0.45
0.65
1.40
2.40
0.47
1.3
2.5
2.6
2.9
1.1
669
170
240
244
---
Desflurane
Sevoflurane
Isoflurane
Halothane
Nitrous Oxide
Relaxant
ED95 (mg/kg)
Succinylcholine
Mivacurium
cis-Atracurium
Rocuronium
Vecuronium
0.1
0.05
0.3
0.05
NM Reversal Agent
Neostigmine
Pyridostigmine
Edrophonium
Drug Infused
Remifentanil
Alfentanil
Sufentanil
Fentanyl
Propofol
Ketamine
Dexmeditomidi
ne
Intubation
(mg/kg)
2 IV, 4 IM
0.3
0.2
0.6
0.15
Dose (mg/kg)
0.05
0.2
1.0
Loading Dose
(ug/kg)
(optional)
1.0 ug/kg
30-100 ug/kg
1-3 ug/kg
2-10 ug/kg
1-2 mg/kg
1-2 mg/kg
0.3 ug/kg over
30 minutes
Emergency Drug
Infusion rate
(ug/kg/min)
N/A
10-20
1-3
10-12
0.6-2.5
Anticholinergic (mg/kg)
Atropine 0.02 or Glycopyrrolate 0.01
Atropine 0.02 or Glycopyrrolate 0.01
Atropine 0.02 or Glycopyrrolate 0.01
Infusion Rate
0.2-0.5 ug/kg/min
100 ug/kg/hr
0.5 ug/kg/hr
10ug/kg/hr
100-200ug/kg/min
25-100 ug/kg/min
0.2 – 0.7
ug/kg/hour
Context-Sensitive
t1/2 after 100 min
3
45
20
220
min
min
min
min
ContextSensitive t1/2
after 300 min
3 min
58 min
35 min
> 270 min
Give loading dose over 30 min.
Redistribution t1/2 6 minutes
Dose
Indication
Phenylephrine
Epinephrine
10 ug/kg (0.1 ml/kg of a 1:100 dilution 1%)
10 ug/kg (0.1 mg/kg of 1:10,000 solution)
Hypotension
Arrest; Anaphylaxis
Na Bicarbonate
(0.3 X kg X base deficit)/2 = mEq
Metabolic Acidosis
Recovery Room Drug
Butorphanol (Stadol)
Morphine
Ketamine
Demerol
Ketorolac
Ondansetron
Metoclopramide
Droperidol
Physostigmine (Antilerium)
Promethazine (Phenergan)*
Diphenhydramine (Benadryl)
Naloxone
Flumazenil
Dose
5-10 up to 30 ug/kg
Up to 0.1 mg/kg divided
0.25 – 0.5 mg/kg
Up to 1 mg/kg divided
0.5 mg/kg
0.1 up to 0.5 mg/kg
0.15 mg/kg
5-25 ug/kg
30 ug/kg
0.25 – 0.5 mg/kg
0l75 – 1.0 mg/kg
1 – 4 ug/kg, titrated
10 – 30 ug/kg
Indication
Analgesia; Sedation
Analgesia
Pain unresponsive to narcs
Analgesia; Shivering
Analgesia
N & V
N & V
Prophylaxis for N & V
General arousal agent
N & V (rescue drug)
N & V, pruritis
Reverse narcotics
Reverse benzodiazepines
* Promethazine (Phenergan) contraindicated in children under 2 years of age as of 4/05
Steroid
Cortisone
Hydrocortisone
Prednisone
Methylprednisolone
Dexamethosone
Amount equivalent to 100 mg
of cortisol
125
100
20
15
1.5
Mineralocorticoid activity
++
++
+++
0
0
For stress-dose steroids, the most typically cited dose is hydrocortisone 2 mg/kg immediately preoperatively
and q6h on the day of surgery. Mineralocorticoid deficiency can be managed by administering saline and
avoiding potassium in IV fluids, but glucocorticoid deficiency should be managed by the administration of socalled “stress dose” steroids to prevent potential cardiovascular depression.
Reference:
Smith’s Anesthesia for Infants and Children, 6th edition. Editors: Motoyama EK, Davis PJ. 1996, Mosby, pp
830-834.
Dosing of commonly used antibiotics:
Ampicillin 50 mg/kg IV
Cefazolin 25 mg/kg IV
Cefotaxime 50 mg/kg IV
Cefoxitin 40 mg/kg IV
Ceftriaxone 50-75 mg/kg IV
Cefuroxime 50 mg/kg IV
Clindamycin 20 mg/kg IV
Nafcillin 25 mg/kg IV
Gentamicin 1.5 mg/kg IV
ENDOTRACHEAL TUBE (ETT) and LARYNGEAL MASK AIRWAY (LMA) DIMENSIONS
Age
premature
newborn
6 mo
1 yr
2 yr
4 yr
6 yr
Endotracheal Tubes
Dia (mm)
Len (cm)
3.0
3.0
3.5
4.0
4.5
5.0
5.5
6 + wgt in kg
10
11
12
13
14-15
15-16
Laryngeal Mask Airways
Patient size
LMA
LMA cuff
size
vol (ml)
babies up to 7 kg
1
2-5
1.5
small child to 20 kg
2
7-10
child 20-30 kg
2.5
15
child > 30 kg
3.0
15-20
normal to large adult
4.0
25-30
ETT diameter must be small enough to allow an audible air leak at < 20 cm H2O.
2.5 mm ETT’s are only used for prematures if a 3.0 will not pass without trauma.
FLUID GUIDELINES
Estimated blood volume (ml/kg):
Age
EBV (ml/kg)
premature
Newborn
Infant < 1 yr
Infant > 1 yr
90-100
80-90
75-80
70-75
Maintenance fluid rates (ml/kg/hr)
First 10 kg: 4
10-20 kg: 2
Over 20 kg: 1
Example: A 17 kg infant requires [(10 X 4) + (7 X 2)] = 54 ml/hr. If he has been NPO 9
hours, his deficit is 54 X 9 = 486 ml. We usually give 1/2 the deficit in the first hour,
then reduce according to maintenance plus expected 3rd space losses for the type of
surgery:
Crystalloid fluids = maintenance + 3rd space + 3 X blood loss
For the following types of surgery, add these ml/kg/hr for 3rd space losses:
eye surgery = 0
hernia = 2
thoracotomy = 4
laparotomy = 6
Example: 17 kg infant NPO 9 hours, for laparotomy: Give 1/2 the deficit (486/2 = 243 ml)
the first hour, then give maintenance (54 ml) plus 6 ml/kg for 3rd space (17 X 6 = 102),
or 156 ml/hr, for the rest of the case. For a 40 ml blood loss, administer another 120 ml
of crystalloid.
Glucose administration should not exceed 7 ml/kg/hr of 5% dextrose (or 6 mg/kg/min) for
extended periods. In infants, a rate of 5 ml/kg/hr of 5% dextrose is sufficient to
protect against hypoglycemia (ref 2).
Estimation of allowable blood loss
allowable blood loss before transfusing = blood volume X {(hct - lowest allowable
hct)/average hct}
ABL = EBV X (hct – lowest hct)/average hct
Example:
3 kg newborn, blood volume 85 ml/kg, hct 30, lowest allowable 24.
EBV = 3 X 85 = 255 ml
hematocrit average = (30 + 24)/2 = 27
255 X (6/27) = 57 ml maximum blood loss, assuming adequate crystalloid/colloid fluid
replacement. (57 ml is about 4 tablespoons.)
1. Weissman C: The metabolic response to stress: an overview and update. Anesthesiology
1990;73:308-327
2. Steward DJ: Fluid management for the pediatric patient. Refresher Course Outline in
Can J Anaesth 1994;41:R87-93.
3. Schreiner MS, Nicholson SC: Update of fluid management for the pediatric patient: from
NPO to drinking postoperatively. Advances in Anesthesia 1995;12:159-181.
Transfusion Considerations in Children
1. For young infants, ask for either relatively new packed RBC’s or washed cells to avoid
hyperkalemia, since older cells may have a very high hematocrit.
2.
For infants, blood should be CMV-negative.
3. For patients likely to be transplanted, or those who will receive chemotherapy, infuse
blood through a leukocyte filter. This will reduce the incidence of FNHTR (febrile
nonhemolytic transfusion reactions) and TRALI (transfusion-related acute lung injury).
This is also an acceptable alternative for preventing CMV infection.
4. Irradiated blood is given to patients susceptible to TA-GVHD (transfusion-related
graft-versus-host disease). Indications include low birth weight infants, patients who
may receive transplants, especially bone marrow transplants, and cancer patients who are
immunosuppressed.
INTRODUCTION TO THE RILEY OPERATING ROOM
NPO ORDERS
Age
< 6 months
> 6 months
Minimum NPO Times for Elective Cases1
Solids/Milk/Formula
Breast Milk
6 hr
4 hr
8 hr
N/A
Clear Fluids
2 hr
2 hr
Easy memory guide is the “2-4-6-8” rule:
2 hours for clears, 4 for breast mild, 6 for formula and 8 for McDonalds.
PREMEDICATION ORDERS
Most children between the age of 1 year and 5 years will benefit from the preoperative
administration of midazolam for anxiolysis. Our standard dose is 0.5 mg/kg not to exceed
15 mg, given 20 minutes preoperatively.
Use midazolam with caution in children with airway problems or CNS abnormalities.
Occasionally patients with autism or other behavioral problems will have unpredictable
responses to midazolam.
14% of children will be “non-responders” to our standard dose of 0.5 mg/kg (Kain et al:
Effects of age and emotionality on the effectiveness of midazolam administered
preoperatively to children. Anesthesiology 2007;107:545-52). Many of these nonresponders are predictable from their behavior (extremely anxious, easily upset,
combative, non-cooperative). Consideration should be given to giving these children a
larger dose of Versed (0.66 mg/kg) or two doses, one an hour preop and another 20 minutes
preop.
Other commonly used oral premedication regimens include:
Clonidine 4 ug/kg (ref: Nishina: Clonidine in paediatric anaesthesia (Review)
Paed Anaesth 1999;9:187-202)
Diazepam 0.2 mg/kg po 1 hr preop
Premedication in children with high risk of aspiration:
H2 blocker: Cimetidine 7.5 mg/kg either IM or PO 1 hr preop
Enhance gastric emptying: Metoclopramide 0.15 mg/kg PO or IV
Antisialogogue: Glycopyrrolate 0.01 mg/kg IM or IV, or atropine 0.02 mg/kg PO
ASA PHYSICAL STATUS
Assignment of physical status is based on the physical condition of the patient,
independent of the planned surgery. It is not a measure of anesthetic risk.
ASA I
ASA II
ASA III
ASA IV
ASA V
ASA VI
E
No organic, physiologic, biochemical or psychiatric disturbance
eg: healthy child
Mild to moderate systemic disturbance
eg: well controlled asthmatic, mild anemia, moderate obesity
Severe systemic disturbance
eg: heart disease that limits activity, cyanotic heart disease, insulindependent diabetic, poorly controlled asthma, marker biochemical abnormalities
(“Wappner Wonders”), patients who have had heart or liver transplants,
prematures with apnea and/or bradycardia
Severe systemic disturbance that is life-threatening with or without surgery
eg: uncontrolled congestive heart failure, advanced hepatic renal or
pulmonary disease
Moribund patient who has little chance of survival with or without surgery
Brain-dead patient for organ harvest
Added to any of the above if emergency operation is required
eg: otherwise healthy 2 year old for testicular torsion would be a IE
eg: otherwise healthy 2 year old with fever, vomiting and dehydration due to
appendicitis would be a IIIE
NEXT DAY’S ASSIGNMENT
To get your assignment for the next day, call 767-7747 for a recording of your room
assignment, first case, start time, and staff. Keep in mind that the schedule at Riley is
very fluid, so things can always change.
CASE START TIMES
All routine cases are to be in the room by 7:30 am, or 9:00 am on Wednesday.
Pumps, spinal fusions, major craniofacial surgeries are to be in room by 7:10 am (9 Wed).
ANESTHESIA TIME
Anesthesia time starts from the time the patient enters the OR until you have finished
gathering vital signs and stabilizing the patient in the recovery room. It is essential
that your records accurately reflect this time, since our billing is based on this time.
These times are audited for accuracy by third party payers.
DAY SURGERY RESIDENT / STAFF
We cover the clinic from 7:30 am to 5 pm every day. Be sure the clinic nurses know your
beeper number. Clinic patients (patients from physicians’ offices for preop workups) take
priority over all other patients. Clinic patients must be worked up within 30 minutes.
Two categories of patients are of special note:
Cardiac catheterization patients must go to Registration, Dental, ECG, ECHO, phlebotomy
and Anesthesia all in one day, so they must be expedited.
Dental patients must have a brief H&P done by us, since dentists cannot do H&P’s. If
the patient has a complex medical problem, you can ask the Pedodontist to obtain a
pediatric consult.
The next highest priority is to see surgical outpatients in a manner best suited to
facilitate the surgical schedule.
Finally, you can facilitate the schedule if you work up inpatients in the holding area who
have not been seen preop. However, the clinic patients and outpatients have priority.
Lunch relief for OR residents should only be done if clinic is caught up; you can never be
unavailable for the clinic for more than 20 minutes.
THE ROC
We currently staff 5 outpatient surgery rooms at the Riley Outpatient Center. The
particular emphasis in the ROC is on efficiency, which is facilitated by additional
nursing staff. Turnover time between cases at the ROC is 3-5 minutes, so plan ahead.
nurses will not ask you if you are ready before bringing a patient back.
The
The men’s locker room code # is 1519, and the women’s code # is 1518.
PREGNANCY TESTING
Pregnancy testing of adolescent females is controversial. In general, testing is not
warranted for females under 15 who are not otherwise suspected of being at risk1. In
addition, many authors feel that the expense and emotional stress of testing (especially
if it reveals an unwanted pregnancy) make testing preoperatively unwarranted, since a
well-managed anesthetic is extremely unlikely to impact on teratogenesis or the rate of
abortion2. In the pregnant teen or teenage female who may be pregnant, consider avoidance
of drugs that have possible teratogenic effects, particularly the benzodiazepines and
nitrous oxide.
If pregnancy testing is undertaken, be sure you have a full understanding of who (the
child and/or the parents) will be apprised of the results before you do the test. If the
minor (under 18 years old) is a parent or is married (ie, she is emancipated), the results
must be reported exclusively to her.
1.
2.
Duncan PG, Pope WDB: Medical ethics and standards. Editorial in Anesth Analg
1996;82:1-3.
Azzam FJ, et al: Preoperative pregnancy testing in adolescents. Anesth Analg
1996;82:4-7.
ADMISSION OF SMALL INFANTS
Infants less than 44 weeks of gestational age must be admitted postoperatively, due to
their increased risk for apnea and SIDS postop. Consider ordering apnea/bradycardia
monitors for very small infants, infants with a history of A&B (apnea/bradycardia),
infants who have undergone placement or revision of VP shunts, or infants who are
otherwise at increased risk for postop apnea.
44 weeks is the lower limit of children that we will consider doing as outpatients. Some
infants who are older than 44 gestational weeks will need to be admitted, even after minor
surgical procedures such as hernia repair. Concise guidelines that cover all reasons for
admission do not exist.
EQUIPMENT AND MONITORS
Complete anesthesia checklist (on clipboard) prior to the first case daily.
NIBP, oximeter, stethoscope, capnograph, ECG and temp probe for all cases.
Emphasize airway-related monitoring, since almost all pediatric morbidity relates to
the airway
POSTOPERATIVE OXYGEN
Children must maintain and airway and SAT > 90% to be transported to the PACU without
oxygen. Once in the PACU, all children who were intubated will receive humidified 40%
oxygen unless you specify otherwise. Huts, masks, nasal cannulas, trach collars and
ventilators are all available.
A highly qualified respiratory therapist (Linda Brearton or John Christopher) is always
assigned to the OR/PACU. They can help you troubleshoot problems with your humidifier,
help with transport ventilation, set up ventilators in the PACU, set up CO2 for certain
cardiac cases, and deliver a variety of treatments pre-, intra- or postoperatively:
Typical treatment regimen:
Racemic epinephrine 0.5 ml in 2.5 ml of NS for postop croup
Ventolin (albuterol) 0.5 ml in 2 ml NS or in Intal (cromoyln sodium)
Alupent (metaproterenol) 0.2 to 0.3 ml in 2 ml NS or in Intal
LIBRARY/COMPUTER ROOM
Access code for library is 49981
ICU RESIDENT
The ICU resident must be thoroughly familiar with all the patients we are following.
Staff must be informed promptly of all new admissions, any major changes, and all
procedures such as extubations, reintubations, line placement.
Your activities must be documented at least daily on the special forms for that purpose,
and staff must sign those forms daily. Our care must be coordinated with that of the
surgical teams, so good communications are essential. A brief note must accompany any
procedure.
Unless otherwise indicated, we follow all postop cardiovascular, orthopedic and
neurosurgical patients admitted to the ICU, many of the burn patients, and some of the
general surgery and trauma patients.
In addition, the ICU resident is responsible for follow up of pain patients – see section
on pain management.
Often the ICU resident at night will need to sedate or otherwise control an infant or
child on a ventilator. Typical drug regimens used for this purpose include:
Fentanyl infusion 2-3 ug/kg/hr
Butorphanol (Stadol) 5-30 ug/kg q 1-3 hrs
Intermittent midazolam (not as infusion) 25-50 ug/kg
cis-Atracurium drips 3 ug/kg/min
Morphine 0.05-0.1 mg/kg intermittently or 20-50 ug/kg/hr as infusion
Chloral hydrate 15-30 ug/kg PO q8h sedative,
Propofol infusions, starting at 40 ug/kg/min
titrating to effect
Dexmeditomidate, load with 0.3 ug/kg over 30
(loading dose is optional and should be done
bardycardia and/or hypotension)
or 20-50 ug/kg PO as hypnotic
(after a 1-2 mg/kg bolus) and
min, then 0.2 – 0.7 ug/kg/hour
with caution, due to the possibility of
The call room is 2175, and the code to enter is 1452.
ICU call beeper is 312-8255.
The password and access codes for the ICU Xrays are both RIPACS.
the nurses station on the west end of ICU South.
There is a viewer behind
At times you will be asked to accompany one of our ventilated patients to CT or MRI or
Xray. Whenever possible, we accommodate nursing by doing this. However, if you are too
busy or have patients that are critically ill or have not yet made complete rounds, you
always have the right to refuse. In that case the surgical service following the patient
must provide the physician escort. If any questions arise on this issue, consult your
staff.
OR CALL RESIDENT
Call staff early when you think a case is pending to be sure lines of communication are
open. If you fail to reach staff on call within 5 minutes, call the back-up staff.
Many emergency cases require arterial lines, so make sure you know where the equipment is
and how to set it up quickly. For complex cases, call in an anesthesia nurse.
The call resident is also responsible for seeing and consenting "add-on" patients for the
next day's surgical schedule. If busy in the OR, have the ICU resident assist with preops. It is essential that cases are not ever delayed due to a lack of anesthesia workup
or permit.
The call room is 2175, and the code to enter is 1452.
ON CALL FELLOW
The fellow on call may take call from home, but is expected to be available within 30
minutes for all neonatal emergencies and other challenging cases. The fellow will also be
called in when the case load is such that a second OR must be opened.
STAFF CALL ROOM
The staff call room is room 4944, just to the right of the elevators as you approach ICU
North. The entry code is Patti’s phone number (5 digits beginning with 4). There is no
telephone.
BEFORE YOU LEAVE FOR THE
All residents must check
that day. The residents
the next day before they
room.
DAY
out with the faculty who is the designated "staff" person for
who finish first are responsible for seeing the inpatients for
leave. A check-off board for inpatients is in the residents'
MISCELLANEOUS
Extubations: Do not extubate children until they are awake, moving all four
extremities, eyes open. Do not take intubated patients to the PACU without first
consulting your staff.
Do not take a patient to the recovery room until your staff has filled out all of the
required paperwork. Missing paperwork = no reimbursement.
Reading in OR: Recreational reading in the OR (newspaper, stock quotes) is verboten.
Some staff allow professional literature (journals, texts) during long cases.
Help: Call for help from your staff any time something is not quite right. Staff are
always rapidly available. Do not wait for a situation to become potentially dangerous
before calling for help.
Staffing cases: We staff ALL cases, period. If you need to staff a case for the next
day, do not hesitate to page or call staff at home.
IV’s: Never stick a patient for an IV more than twice without getting staff
assistance. Check IV’s from the ward very carefully before use. Switch ward IV’s to
OR fluids prior to induction.
Broken equipment: Never leave a piece of malfunctioning equipment in the OR. If a
piece of equipment cannot be fixed, be sure nursing or medical engineering removes it.
Riley nursing staff: Treat our nurses with respect. Most of them are specialists in
the type of cases they scrub, most have been here a lot longer than you have, and they
are dedicated to the welfare of our patients. If any problems arise between you and
nursing personnel, it is staff responsibility to resolve the conflict.
2/06
TWolfe
PHONE NUMBERS
STAFF
Allison, Jackie
Carvallo, Dan
Emhardt, John
Green, Mort
Hardacker, Doris
Johnson, Jodi
Kibby, Brandon
Krishna, Gopal
Kritzmire, Stacy
Latham, Leigh
Mazurek, Mike
McNiece, Bill
Nouri, Malik
Presson, Rob
Presto, Eugene
Saysana, Chansamone
Sheplock, George
Stasic, Andy
Tolley, Jim
Vetter, Tom (pain)
Walker, Scott
Wolfe, Tom
PAGER
312-2228
312-2063
312-1952
312-1954
312-1955
312-1961
312-1962
312-1964
312-2473
312-1966
312-1968
312-1969
312-2729
312-1975
312-2702
312-1698
312-1980
312-1981
312-2596
312-2534
312-2604
312-2606
PHONE
873-8736
468-1719
823-9292
587-1050
571-8062
257-5660
858-7778
293-7765
322-1794
594-9057
254-0296
253-5712
334-0382
255-3079
1-773-320-1713
826-8816
733-0152
873-5168
837-1451
570-9287
388-0413
326-8555
FELLOWS
Bowlen, Michelle
Makino, Kotaro
312-2502
312-1022
259-8137
201-5389
NURSES
Stephanie Whittaker(pain nurse)
Anesth Nurse Main (Cathi Evans)
Anesthesia Nurse ROC
Periop Nurse Practitioner
(Matti Upano)
OTHER NUMBERS
Patient Information
Assignments for next day
OR resident on call
ICU resident on call
Recovery Room
Riley OR Main Desk
Riley charge nurse
Any Riley OR
Day Surgery
Residents’ Room
Medical Engineering
Riley Secretary Patti Liggins
Fesler Office
Anesthesia Workroom RI
Cardiac Cath Lab
XRay, RI
CAT scan RI
MRI RI
MRI site 1
MRI tech room
MRI site 2 (cardiac MRI)
Radiation Therapy
Radiation Therapy (Rx room)
ERCP Lab UNI
XRay UNI
312-2324
312-8225
312-8259
4-8244 or 4-8810
767-7747
312-1978
312-8255
4-9945 or 4-9947
4-8222
cell 278-8102
4-9950 + rm # (rm 9 = 49959)
4-9997
8-5874
4-7140
4-9981
4-0275, 4-0269, or 4-0273
4-9978
4-2612
4-7804
8-6342
4-2566 or 4-9881
8-4422
8-4417
8-4469
4-1181
4-1301
4-4837
4-2621
XRay Specials UNI
Ablation Lab UNI
University OR Main Desk
Anesthesia Workroom UNI
Staff Call Room Rm # 2175 G
ROC admitting desk
ROC OR’s
ROC scheduling
Clinical Engineering
Lab: Coagulation
Lab: Chemistry
Lab: Hematology
Lab: Vital Funtions
Lab: Specimen receiving
4-4831
4-8024
4-4001
4-2722
4-3180
8-1511
8-150 + rm # (rm 2 = 8-1502)
8-1484
4-4376
4-1764
4-1760
4-1763
4-5381
4-4542
Clarian North phone numbers are as follows:
Main Hospital Number 688-2000
Night Administrator pager 312-9621
Posting of OR cases, schedule changes, after hours emergency cases 312-9655
OR Charge 8-2096
PACU 8-2165
POC 1 8-2104
POC 2 8-2141
PAT 8-3177
Surgery WR 8-2099
OR Scheduling 8-2100
Pharmacy OR 8-2783
Bed Control 8-3034
ER 8-3139
NICU 8-2355
Peds 8-2400
Blood bank 8-2756
Prot. Srvices 8-2911
Radiology 8-3058
CT MRI 8-3054
Cath lab 8-3060
PAIN MANAGEMENT
AVAILABLE SERVICES: Special methods of postoperative pain control, including PCA's and
epidural infusions of either local anesthetics or narcotics, and caudal blocks with 0.25%
bupivacaine or 0.2% ropivicaine, or ilioinguinal and iliohypogastric blocks for hernia
repairs, are offered to a wide variety of patients. Dr Vetter has detailed protocols
available for these services, which are continously updated. Followup of pain patients
will be the responsibility of the ICU resident. All epidural injections and PCA changes
must be staffed with an attending anesthesiologist. Consider epidural morphine in
patients who have major abdominal, thoracic or extremity surgery, and consider epidural
narcotics for patients with lower abdominal or extremity surgery. Consider PCA for
patients of school age or older. Consider constant narcotic infusions only for patients
who will be in the ICU. Toradol 0.5 mg/kg q6h can augment many pain regimens without
serious side effects. Avoid toradol in orthopedic patients (bleeding), and limit its use
to 4 days.
Epidural Narcotics: Place epidural catheter and load intraoperatively with 50 ug/kg of
morphine. Have pharmacy prepare morphine for epidural infusion (5 mg/250 ml for patients
< 25 kg, 10 mg/500 ml for larger patients) and run at an initial rate of 0.2 ml/kg/hr.
EPIDURAL OR CAUDAL NARCOTICS
DOSES:
Morphine 0.05 - 0.1 mg/kg (usual dose 0.07 mg/kg); duration 12 hrs
Fentanyl 1 mcg/kg, duration 3-4 hours
SIDE EFFECTS:
Respiratory depression, usually 4-8 hrs after MS, more rapidly after fentanyl,
resolving by 24 hours.
Pruritis
Nausea/vomiting
Urinary retention
TREATMENT OF SIDE EFFECTS:
Naloxone, 0.5 - 1.0 ug/kg followed by 0.5 - 1.0 ug/kg/hr. Effective for treatment of
respiratory depression and pruritis, but less effective for N&V, urinary retention.
Will not reverse analgesia if titrated.
Expected Side Effects of Epidural Narcotics:
Respiratory depression: usually 4-8 hours after MS, more rapidly after fentanyl,
resolving by 24 hours. Often associated with concomittant administration of
intravenous narcotics.
Pruritis
Nausea/vomiting
Urinary retention
Treatment of Side Effects of Epidural Narcotics:
Naloxone, 0.5-1.0 ug/kg followed by 0.5-1.0 ug/kg/hr. Effective for treatment of
respiratory depression and pruritis, but less effective for N&V, urinary retention.
Will not reverse analgesia.
Metoclopramide 0.06-0.1 mg/kg may be useful for treatment of N&V in some cases.
Typical Doses for Epidural Narcotics:
Epidural MS: 0.05-0.1 mg/kg, duration up to 12 hrs. Frequently used via caudal route
for open heart cases.
Epidural Fentanyl: 1 mcg/kg, duration 3-4 hours.
NMDA receptor antagonists (ketamine, magnesium)
In double-blinded studies in children, ketamine and/or magnesium have not been shown to
reduce postoperative pain or analgesic requirements in children (ref 3 & 4)
PCA'S :
Loading dose as necessary to relieve pain, usually about 0.05-0.1 mg/kg morphine
PCA dose ("incremental dose") 0.025-0.04 mg/kg
Lockout interval: 10-20 minutes
Continuous infusion rate: 0.02-0.05 mg/kg/hr
Four hour limit: 0.25 mg/kg
No parenteral narcotics should be ordered by other services during PCA
Rx N&V: Prochlorperazine (Compazine) 0.1 mg/kg po or pr (children > 10 kg only)
Rx Pruritis: Diphenhydramine (Benadryl) 1 mg/kg po or 0.5 mg/kg IV q6h
KIDDIE CAUDALS:
Goals: Intraoperative and especially postoperative analgesia
Indications: GU, herniorrhaphy, rectal procedures and lower extremity surgery
Age: Caudals are used in patients from newborn to adult. However, controversy exists
regarding possible toxicity in infants, and therefore currently we do not routinely
perform caudals on infants less than 1 month of age (arbitrary cutoff).
Also, as
children approach 7-8 years, accessing the caudal space becomes more difficult and a
higher failure rate should be anticipated.
Preoperative Visit:
Specific informed consent should be obtained for caudal blocks
Cover these specifics with the parents:
Describe the technique (useful analogy is epidural block for labor)
Stress that the child will be asleep when the block is performed
List the advantages (mainly postoperative analgesia)
Describe possible complications: subcutaneous injection with inadequate analgesia,
intravenous or intraosseous injection (<1%), dural puncture (extremely rare except in
very small infants). See ref 1. If a narcotic will be added to the epidural
anesthetic, mention must be made of the possibility of respiratory depression.
Discuss expected duration of block (6-8 hours). Advise parents to begin analgesics
prescribed by surgeon at first sign of discomfort.
EQUIPMENT
Caudal kits are available. These contain 0.25% bupivacaine (30ml), epinephrine (1 mg),
sterile flats, a 1 ml and a 10 ml syringe, sterile drapes, a bandaid, and a 21 gauage
butterfly needle.
Epinephrine: add 0.15 ml of epinephrine solution to the 30 ml of local anesthetic to
make a 1:200,000 solution, if you intend to use epinephrine. If you are using
ropivicaine as your local anesthetic, add 0.10 ml epinephrine to the 20 ml vial of
ropivicaine.
Draw up the anticipated dose of local anesthetic plus 3 ml to flush the butterfly and
to allow for some extra for wastage in case of inadvertent subcutaneous injection on
first attempt
DRUG/DOSE
By weight: From 0.5 ml/kg for GU procedures (T10 level) up to 1.0 ml/kg for upper
abdominal surgery (T4 level), to a maximum of 15-20 ml.
By segment blocked: 0.056 ml/kg/segment. For example, for T10 level, 5 sacral + 5
lumbar + 2 thoracic segments = 12; therefore, dose in 10 kg child = 0.056 X 10 X 12 =
6.7 ml.
Clonidine: we usually add clonidine 1 ug/kg for outpatients, and 2 ug/kg for
inpatients.
TECHNIQUE
When: The block is usually performed at the beginning of the procedure if the surgeon
operates by the clock and not the calendar, or may be performed at the end of the
procedure. If a block has been performed at the beginning of a procedure that lasts 3
or more hours, consider reblocking at the end with about 2/3 of original dose.
Position: Position patient left side down if you are righthanded, reverse if you are
lefthanded.
Landmarks: Define landmarks (coccyx, sacral cornua and the sacral hiatus covered by
the sacro-coccygeal ligament) prior to prep.
Prep: Prep area over hiatus with Betadine. Use small circular strokes, then wipe
downward toward gluteal fold and discard. Repeat X 3. Never wipe back over the sacrum
after the prep spoinge has been near the gluteal fold. Blot any liquid Betadine
remaining to eliminate the possibility of Betadine entering caudal space.
Drape area, or at a minimum lay a sterile drape on table under patient to give yourself
a sterile field on which to place objects.
Retract skin over sacral hiatus with left hand, and insert butterfly at about 45 degree
angle, with the needle bevel down (needle “upside down” -- this makes interosseous
injection less likely).
A definite "pop" or loss of resistance to injection is felt
as the sacro-coccygeal ligament is pierced. At this point, flatten the angle of the
needle so that it is parallel to the axis of the spine and advance 1-2 mm and no more.
Attempt to aspirate for blood or CSF.
Inject 1 to 2 ml test dose and observe for tachycardia as sign of intravenous
injection. Give the balance of the dose slowly to reduce severity of possible toxic
reaction in case of negative test dose. Solution should inject very easily, with no
resistance.
Wash the Betadine prep off of the skin and cover injection site with Bandaid.
TOXICITY
Since there is great potential for cardiac toxicity with bupivicaine, we routinely use
ropivicaine. Toxicity from bupivicaine in the anesthetized infant usually manifests as
cardiovascular toxicity. The toxicity can be heralded by markedly elevated ST-T
segments, or more commonly by a wide-complex tachycardia rapidly progressing to
cardiovascular collapse. Prolonged CPR may be required, and cardiopulmonary bypass
should be considered when resuscitation is not rapidly effective.
ILIOHYPOGASTRIC/ILIOINGUINAL BLOCKS:
Drug: 0.5% bupivacaine, 1.25 mg/kg = 0.25 ml/kg, or 0.25% bupivacaine, 0.5 ml/kg, or
equivalent dose of ropivicaine.
Insertion of needle 1 cm medial and superior to anterior superior iliac crest, directed
downward and outward until it strikes inside of ilium, then anesthetic injected as
needle withdrawn. Needle reinserted and directed toward inguinal ligament (definite
"pop" is felt when external oblique fascia pierced); local then injected in fan-like
pattern in the direction of the symphysis pubis.
Block usually performed at conclusion of surgery to avoid obscuring surgical landmarks
REFERENCES
1. Dalens et al: Caudal anesthesia in pediatric surgery: Success rate and adverse
effects in 750 consecutive patients. Anesth Analg 1989;68:83-9
2. Eyres RL: Local anaesthetic agents in infancy (Review article). Paediatric Anaesth
1995;5:213-218.
3. O’Flarerty JE, Lin CX: Does ketamine or magnesium affect posttonsillectmoy pain in
children? Paediatric Anaesthesia 13;2003:413-421
4. Dix P, et al: Double-blinded randomized placebo-controlled trial of ketamine on
postoperative morphine consumption in children following appendicectomy. Paediatric
Anaesthesia 13;2003:422-426.
Last updated 03/04
TWolfe
MAGNETIC RESONANCE IMAGING
ANESTHETIC PROBLEMS DURING MRI
High strength magnetic field disables most electronic monitors
During active imaging, a very loud "drumming" sound makes it difficult to hear
respiratory and cardiac sounds
The patient is nearly inaccessible within the imaging cylinder
Metallic objects can become missiles. This includes laryngoscope handles and blades
Watches, credit cards, etc are destroyed
Certain patient-associated objects may be affected, eg pacemakers, aneurysm clips
The DC lighting system in the MRI unit is barely adequate to observe the patient
The MRI unit is kept at a low temperature
ANESTHETIC MACHINE
We have an anesthetic machine from which nearly all ferromagnetic materials have been
removed, and that can therefore be used in the imaging room. Note that the gas
cylinders on the side of the machine are special aluminum tanks.
We also have special MRI-compatible laryngoscope handle and blades.
CURRENT MONITORING
In the induction room, you should have all the normal monitors routinely available in
the OR. When you transition from the induction room to the MRI room, your choice of
monitors will be severely restricted:
- BP: A special cuff and BP monitoring unit are available in the MRI room. Arterial
lines cannot be monitored
- An accurate end-tidal monitor is now available. Use low-dead-space adapters for
infants < 10 kg
For spontaneously ventilating patients, the motion of the bag can be an important
respiratory monitor
Pulse oximetry: A special pulse oximeter unit is available for use in the MRI room.
It has a large clip that occasionally occludes flow over time in infants’ toes or
fingers, degrading the signal.
Temperature: No continuous temperature monitor can be used inside the MRI room. A
mercury thermometer can be used
ECG: ECG is not generally monitored during MRI scanning. The wires themselves will
interfere with some scans, so remove the wires and metal-containing patches from the
patient before entering the MRI scan room.
Heart and Breath Sounds: Make sure you use only an MRI-compatible stethescope.
A digital clock is located on the oximeter/BP monitor.
In most cases, you can watch the monitors from the window outside of the scan room,
thus saving your ears from the prolonged “drumming” of the scanner.
ANESTHETIC TECHNIQUE
In general, any anesthetic technique appropriate for the child's condition can be used.
Anesthesia is first induced outside the imaging suite, then the child moved into the
scanner under anesthesia. In most cases, spontaneous ventilation is maintained.
In the majority of cases, anesthesia is induced by mask outside the imaging room. Then
an IV is established, and an airway (either LMA or ETT) is established after a small
bolus of propofol. Spontaneous ventilation re-established, and the case done with the
child spontaneously breathing an inhaled anesthetic.
If the patient has a good airway and is relatively healthy, a propofol induction of 2
mg/kg followed by a propofol drip of 80-120 ug/kg/min will keep most children still
(ref 6 & 7).
Emergence from general anesthesia is often delayed until the patient is moved outside
the imaging room so that you can awaken the patient where you can use all of our normal
equipment and monitors. Also, it is important to clear the MRI room quickly to allow
for continuous use of the scanner. A recovery area is located just outside the
scanner. The last case each day is transported to the OR recovery room, since the MRI
recovery nurses will not manage patients without anesthesia staff present.
Infants should be wrapped in bubble wrap and a soft bunting to conserve body heat.
However, excessive insulation is not necessary, as the radiofrequency radiation
generated by the scanning process tends to warm patients (ref 7).
Artificial noses (devices to conserve airway humidity) are available. This is
important, in that the heated humidifier cannot be used in the MRI suite.
EMERGENCIES
Emergencies: If a crisis arises during a scan, remove the patient from the imaging
room and resuscitate outside. The reasons for this are (1) shutting down the magnets
is a very expensive procedure that technically needs to be avoided if at all feasible,
and (2) your equipment for resuscitation (defibrillator, monitors, laryngoscope, etc)
will not function in or cannot be taken into the MRI imaging room.
CARDIAC MRIs
For some vascular MRIs, ventilation must be periodically held for up to 30 seconds at a
time, so ventilation must be controlled in these patients.
START TIMES
Efficiency is essential in the MRI unit, since it is usually booked solid. The
scheduled time is the time at which the patient is to enter the scanner, not the time
your anesthetic is to start, so plan ahead.
EQUIPMENT NOTES
The humidifier cannot be plugged in during the scan. Even in the "off" position, the
electric connection interferes with the scan image. Currently, we usually leave the
humidifer out of the circuit.
An anesthesia nurse is assigned to all MRI cases. She will be able to help you locate
any equipment that you need.
PHONE NUMBERS
MRI: 42566
Recovery Room:
49945
Day Surgery:
49997
OR:
48222
CONTRAST MEDIUM
The contrast medium that is used to enhance some of the MRI’s is Magnevist, or
gadolinium. It is a non-iodine-containing so reactions are rare. Injection rate is
supposed to be limited to 10 ml/min.
REFERENCES
1. Weston G, et al: Imaging for anaesthetists: A review of the methods and
anaesthetic implications of diagnostic imaging techniques. Can Anaesth Soc J
1985;32:552-61.
2. Nixon C, et al: Nuclear magnetic resonance. Its implication for the anaesthetist.
Anaesthesia 1986;41:131-37.
3. Roth J, et al: Patient monitoring during magnetic resonance imaging. Anesthesiology
1985;62:80-83.
4. Rao CC, et al: Modification of Ohmeda Excel 210 anesthesia machine for use during
magnetic resonance imaging. Anesthesiology 1989;71:A365.
5. Frankville D, et al: The dose of propofol required to prevent children from moving
during magnetic resonance imaging. Anesthesiology 1993;79:953-958.
6. Barst S, et al: A comparison of propofol and chloral hydrate for sedation of young
children during magnetic resonance imaging scans. Paediatric Anaesthesia 1994;4:24347.
7. Bryan YF, et al: Brain magnetic resonance imaging increases core body temperature in
sedated children. Anesth Analg 2006;120:1674-9.
Updated 6/06
TWolfe
RADIATION THERAPY
Many infants and young children undergo radiation therapy for a variety of malignancies,
particularly retinoblastomas and CNS tumors. Unfortunately, many of these children are
too young to be cooperative. Therefore, even though each daily treatment involves only 2
to 3 painless exposures, and each exposure lasts less than 90 seconds, an anesthetic is
necessary to assure immobility.
Two major considerations complicate the anesthetic management of these infants. First,
the anesthesiologist cannot be in the room with the patient during the treatment period,
since he would be exposed to toxic levels of radiation. Therefore, the airway must
somehow be maintained even though the anesthesiologist cannot be physically present, and
adequate monitoring must be assured during the time that the anesthesiologist is out of
the treatment room.
Second, a series of 20 to 30 daily treatments is typically required, and therefore these
infants must be made NPO daily for weeks at a time. If recovery and the ability to feed
is not quickly restored, these patients can suffer from dehydration and starvation.
Therefore, agents that have a long recovery time (eg, IM ketamine) are relatively
contraindicated.
After considerable experimentation with various IV, IM and inhalation anesthetics, with
and without intubation, we have evolved a very simple and effective anesthetic technique
for dealing with these patients. Following an inhalation induction with sevoflurane and
nitrous oxide/oxygen, these children will maintain an adequate airway under
sevoflurane/oxygen anesthesia for the brief period of the treatments. Therefore, after
anesthesia is induced with mask sevoflurane, the patient can positioned for the radiation
treatment port, and then left breathing spontaneously while the anesthesiologist exits the
treatment suite. Outside the suite, the anesthesiologist monitors the child by observing
chest excursions on a video monitor, by observing the waveform and digital output of a
pulse oximeter on a second video monitor, and by listening to an ECG heart tone on an
audio monitor. If during any part of the treatment the anesthesiologist is concerned
about airway patency or other parameters, the treatment can be instantly aborted and the
anesthesiologist can immediately re-enter the treatment suite.
Currently, propofol is usually the agent of choice in those patients with a central line.
Typically dose rates of 200 ug/kg/min are required, at least initially (1). Another
alternative, especially in children who do not tolerate propofol well, is methohexital,
initiated with 1-2 mg/kg boluses, then maintained with a drip at 200 ug/kg/min (2).
If the patient must be positioned prone, the patient is turned
body mold. The head position of the mold has cutouts for the
Plastic sheeting is draped around the head portion of the mold
vapors. Sevoflurane and oxygen are then insufflated under the
into a special head and
patient’s nose and mouth.
to contain anesthetic
plastic sheeting.
Treatments are scheduled early in the day for these patients so that their breakfast is
only minimally delayed. They are kept NPO for solid foods for 6 hours pretreatment, but
are allowed clear fluids up to 2 hours before the treatment.
Recovery from these very brief daily anesthetics is uniformly rapid and smooth, allowing
children to be returned to the care of their parent within about 15 minutes of the end of
the procedure. Recovery room services are seldom required except on days when prolonged
“set-up” procedures are performed.
CHECKLIST:
Be sure oxygen and nitrous tanks are adequately filled, since there are no wall sources
of gases.
Check anesthetic circuit. The same circuit and mask can be labeled for the patient and
reused.
Check laryngoscope, ETT, suction. The suction in some rooms is a portable Gomco unit.
Drugs: thiopental or propofol, succinylcholine drawn up and ready.
Monitors: pulse oximeter, ECG, NIBP, ET CO2.
usually not used.
IV available.
Temperature monitor available, but
TOTAL BODY IRRADIATION
Prior to receiving stem cell transplants, patients must undergo total body irradiation
(TBI). TBI consists of 3 or 4 days of irradiation, 3 times each day, for a total of 9 to
12 sessions. Each session takes roughly 30 minutes, and may involve position changes,
including turning the patients prone.
Sessions are scheduled 4 hours apart, beginning at
8 or 9 am. All of these patients have central lines in place.
Very young children (18 months and less) can for the most part be immobilized for their
treatments. Older children do not require sedation, since the irradiation is painless.
However, children in the 2 to 5 year old age group may occasionally require anesthesia.
Most likely, a propofol infusion via their central line will be appropriate.
Anesthesia will be responsible for the NPO orders for these patients. The guidelines we
have given radiation therapy are 6 hours for solid foods, 3 hours for clear liquids,
though you may consider more liberal clear fluid orders, depending on the case, since
otherwise these children will be NPO all day long for 4 straight days. However, many of
the patients have a total loss of appetite with onset of the treatments, and are
hyperalimented. When these patients are first seen, permits should be obtained for the
entire series of treatments. Also, NPO orders for the full 4 days should be written.
A propofol infusion is the usual anesthetic technique. Typical sequence of events:
Before patient enters treatment room, you should put on a mask, since these kids are
immunosuppressed.
Set propofol pump to deliver boluses of 500 ug/kg.
As soon as the patient is brought into the treatment area, hook the IV, with propofol
attached, to patient’s central line. Be careful to use a click-lock and careful
sterile technique.
Begin propofol boluses until patient becomes somnolent and cooperative. Typically 3 to
4 mg/kg of propofol is required initially to keep patients still enough to tolerate
positioning. Then begin a constant propofol infusion of 200 ug/kg/min initially.
Place pulse oximeter, nasal oxygen cannula (1 to 2 L/min) with end-tidal CO2 port, and
NIBP cuff.
Position patient on gurney. Every other treatment is done either prone or supine.
Attach ECG wires after positioning. Temperature monitoring is optional. Be sure that
there is sufficient slack in the IV tubing, nasal cannula tubing, and monitoring wire
to accomodate the movement of the patient gurney, which will slowly move approximately
the length of the patient under the treatment port.
Be sure that TV cameras are aimed at the monitor and at the patient, since you will
monitor the majority of the case from outside the treatment room.
The treatment usually lasts about 30 minutes, but can be interrupted at any time to
allow you to enter the room.
Recovery is usually very rapid. The child is turned back over to the parents and the
acompanying nurse soon after anesthesia is terminated. Parents usually spend all day
in the radiation suite with their child during treatment days to minimize infectious
exposure.
A good contact person for problems is the transplant coordinator nurse, Connie Stuckwisch,
at 4-3304. Another person to contact is Dr. Frank Smith, pager 3221, one of the radiation
oncologists.
References:
1. Aldrige LM, et al: Propofol infusion for radiotherapy. Paediatr Anaesthe 1992;2:133.
2. Metriyakool K: Methohexital as alternative to propofol for IV anesthesia in children
undergoing daily radiation treatment: a case report. Anesthesiology 1998;88:821-2.
updated 2/06
TWolfe
GASTROINTESTINAL ENDOSCOPIC PROCEDURES
PATIENT EVALUATION: Many of the patients for gastroscopy or esophagoscopy are at risk for
aspiration, due to altered motility, increased gastric acid secretion, or GI bleeding.
Patients for colonoscopy are more likely to have electrolyte disturbances. Either group
may be anemic.
SURGICAL CONSIDERATIONS:
Patients may need to be rolled into a variety of positions to facilitate passage of,
especially, the colonoscope.
Occasionally the endoscopist will need you to produce bowel atony for short periods of
time. This can be accomplished by giving glucagon in small doses. In adults, 0.1 mg
of glucagon will produce bowel atony lasting about 3-4 minutes. A 1 mg dose lasts
around 15 minutes. Doses above 1 mg may result in nausea. It is probably prudent to
run a glucose-containing solution during use of glucagon and for a while post-op.
You will also be asked on occasional to give secretin for pancreatic stimulation. This
is administered over one minute.
For colonoscopies with ARM (anal-rectal manometry) avoid muscle relaxants and
anticholinergics.
For UGI’s with pH probes, only give antiemetic medications (ondansetron, decadron)
after consulting with endoscopist. Avoid anticholinergics.
ANESTHETIC CONSIDERATIONS:
Aspiration prevention: Many patients for UGI endoscopy are at high risk for
aspiration, so appropriate precautions need to be observed. Most of the patients with
reflux can be safely induced by mask, but occasional patients are exceptions.
Positioning: Position patients for either endoscopic procedure in left lateral
decubitus (right side up), with an axillary roll to prevent brachial plexus injury.
Patients should remain supine after induction until the endoscopist has had an
opportunity to examine the abdomen.
Gastric Evacuation: Avoid gastric suctioning prior to gastrostomy or esophagoscopy to
avoid traumatizing the mucosa prior to the endoscopic exam.
Thermoregulation: Smaller patients may become hypothermic during long endoscopies.
For longer exams in small patients, consider placing a chemical heat pad under the
patient. Rarely, you might resort to a Bair Hugger. Warming lights cannot be used,
since the room lighting must be kept low for the endoscopist.
Esophageal Varices: Patients with portal hypertension may present for endoscopic
injection of a sclerosing solution into esophageal varices. The injected sclerosing
agent can induce some degree of bronchospasm and pulmonary pneumonitis.
Intubation: Usually facilitated with mivacurium, so that reversal is not necessary.
Maintenance: A sevoflurane or desflurane anesthetic, with or without a propofol drip,
is our standard anesthetic. A propofol drip has the advantage of reducing the amount
of inhaled anesthetic, which generally results in a smoother and more rapid emergence,
plus it reduces postop nausea and vomiting. Typically propofol is run at 50 ug/kg/min,
and continued right to the end of the case.
Emergence: Patients may be awakened on their side, in the position used for the
endoscopy. This is a particularly good emergence position for patients at risk for
aspiration.
PERCUTANEOUS LIVER BIOPSIES
PATIENT EVALUATION: Obviously, patients undergoing liver biopsy are at risk for
hepatocellular disease and clotting abnormalities.
ANESTHETIC CONSIDERATIONS:
- An immobile diaphragm is necessary when the surgeon advances the biopsy needle to avoid
the possibility of a pneumothorax. Therefore, hold the breath of a patient who is
ventilated, or in the patient under mask or LMA, gently hyperventilate them to the point
of transient apnea before the biopsy needle is inserted. Alternatively, administer a
small dose of succinylcholine.
- Use non-glucose containing IV fluids, since the presence of glucose will significantly
alter the interpretation of some liver biopsies.
- Before starting, blood must be T&C’d.
1/06
Twolfe
References:
1. Schwartz DA, et al: Gastric contents in children presenting for upper endoscopy.
Anesth Anlag 1998;87:757-60. Conclusion: acidity and volume no different than normals.
SELECTED ENT SURGERIES
TONSILLECTOMY
While the most common indication for tonsillectomy is chronic pharyngitis, many children
scheduled for this procedure have an airway-related problem. These children often have a
history of obstructive sleep apnea (OSA), or severe CP with marginal airways, or have some
physical restriction of the airway, such as micrognathia in the Pierre Robin anomalad.
Therefore, their airways are at risk both during induction and especially during
emergence. Some children may even have cor pulmonale or pulmonary hypertension.
Anesthetic Considerations:
Be conservative in your use of premedication. Midazolam often has its maximum effect
just when you are ready for emergence. This can both prolong emergence and, in
children with marginal airways preoperatively, lead to airway obstruction after
extubation. If children with a history of sleep apnea have received premedication with
midazolam, consider reversing it with 30 ug/kg of flumazanil prior to emergence.
When inducing intravenously, consider using propofol, both for its brief duration and
for its antiemetic effects.
Use prebent oral RAE endotracheal tubes, often one size smaller than you would normally
use, since they have large bulky cuffs.
To keep blood from being aspirated around the ETT, use cuffed tubes or tubes that have
a minimal leak. Inflate cuff to "just seal". Keep a small amount of positive pressure
(eg, 4 cm PEEP) in the airway to prevent blood or secretions from trickling down by the
ETT. Always have a spare ETT at hand in case surgeon inadvertently extubates patient.
Considering limiting your FiO2 if the surgeon is using electrocautery on the tonsillar
bed.
Be sure the surgeon removes the throat pack prior to extubation.
Inhalation agents are chosen to insure prompt recovery to pre-anesthetic state.
Sevoflurane is good, since emergence is rapid after short cases. “Bucking” is very
common if desflurane is used.
A propofol drip can be used to supplement your anesthetic. It reduces your need for
inhaled anesthetic, facilitates a smoother and more rapid emergence, and reduces postoperative pain. Propofol is generally set to run at 50 ug/kg/min, with a bolus setting
of 500 ug/kg.
Use a very short-acting non-depolarizer for paralysis for intubation (eg, mivacurium),
or intubate deep without a muscle relaxant.
Extubate only when fully awake. Be prepared to re-establish the airway. Postoperative
respiratory complications occur in about 20% of cases (ref 9), and are most likely in
children who had a poor sleep study preoperatively, or who are less than two years of
age, or have a concurrent medical condition.
To prevent the consequences and lessen the incidence of post-operative nausea, hydrate
well, treat pain appropriately, consider ondansetron 0.15 mg/kg after induction (ref
1), and give dexamethasone 1 mg/kg (maximum dose of 20 mg) intraoperatively (ref
2,3,4,7). Interestingly, maintaining a high FiO2 (80%) during surgery also provides
prophylaxis against postoperative nausea and vomiting (ref 8).
Be sure parents understand that postoperative pain control is never perfect. Let them
know that we will be titrating pain medications, balancing analgesia against apnea.
Pain can be treated with incremental doses of morphine or butorphanol (Stadol), but
narcotic-based drugs (including Stadol) must be titrated carefully in children at risk
for obstruction and airway obstruction. Intraoperative dexamethasone, in addition to
its antiemetic properties, greatly reduces postop pain as well (ref 10). Children with
OSA (obstructive sleep apnea) are known to be very sensitive to narcotics (ref 12).
In addition to the dexamethosone (1 mg/kg to max of 20 mg), Dr. Matt wants ampicillen
given in the dose of 40 mg/kg, maximum of 1 gm.
Ketorolac is not recommended for postoperative analgesia, due to its propensity to
cause bleeding (ref 5).
NMDA-receptor antagonists (ie, Ketamine and/or magnesium) failed to demonstrate a
decrease in pain scores or analgesic requirements following tonsillectomy. (ref 11)
If you do use ketamine as part of your anesthetic technique, be sure to limit the dose,
usually to 0.5 mg/kg.
POST-TONSILLECTOMY BLEED
Severe post-tonsillar bleeding is a rare, life-threatening complication. Management
includes rapid infusion of volume (crystalloid and 5% albumin until blood available) and
rapid re-establishment of the airway so that surgeon can get access to the bleeding site.
To reintubate:
Turn patient to side, empty stomach with large-bore oral gastric tube.
Preoxygenate
With large bore suction immediately at hand, do rapid sequence induction with cricoid
pressure, usually using succinylcholine as the relaxant.
If aspiration of blood has occurred, either during or before intubation, you will see
the classic picture of any gastric aspiration: decreased SAT’s, bronchospasm, high
airway pressures, and chest infiltrates especially on the right. Fortunately, blood
aspiration usually responds well to therapy (PEEP, bronchial toilet, bronchoscopy,
bronchodilators), classically clearing within 12-48 hours.
EAR SURGERY
Patients tend to be nauseated postoperatively due to vagal stimulation from the ear
canal or due to disturbances of the labyrinth. Keep well hydrated, use ondansetron
0.15 mg/kg prophylactically, provide adequate analgesia, and avoid sudden direction
changes when transferring the patient by cart to the PACU.
- Tympanomastoids: Do not keep patient paralyzed, since surgeon may need to locate the
facial nerve using a nerve stimulator.
Tympanoplasties: Surgeon may wish N2O discontinued while a tympanic graft is being
laid on, as N2O bubbles may tend to lift graft off of the desired position.
Cochlear Implants: Do not paralyze these patients, since the surgeon may test facial
nerve function during the case.
BILATERAL MYRINGOTOMIES & EAR TUBES (BMT’S)
Many Riley patients for BMT's will be ASA III patients, so be sure you work them up the
same as any other patient. Most are done with sevoflurane by mask, with no IV. The
"secrets" of good mask technique are:
Elevate the jaw by placing your fingertips gently behind the angles of the mandible,
rather than wrapping your hand around the mandible as you would for an adult.
Use both hands to symmetrically hold mask and jaw.
Be gentle. Do not overly stimulate patient under light plane of anesthesia by
forcefully manipulating the jaw.
For mask anesthesia in small children, the thumbs hold the mask in place by application
of firm pressure over the bridge of the nose, and the ring fingers elevate the jaw by
application of pressure behind the angle of the mandible. The other fingers are not
used. In particular, the fingers must not curl around the mandible, applying pressure
to the base of the tongue and obstructing the airway. Note head in the ideal position
for either intubating or mask anesthesia -- the "sniffing" position, with neck flexed
and atlantoaxial joint extended.
Controversy exists over the need for post-op pain medications in BMT’s, but one way to
deal with the issue is to give Tylenol suppositories while the infants are asleep. This
obviates the nurse in PACU having to try to give Tylenol to an emerging, upset infant.
The three sizes of aceteminophin suppositories are 80, 120 and 325 mg.
For patients 10 to 40 kg, give a small (80 mg) suppository
For patients 40 to 60 kg, give a medium (120 mg) suppository
For patients over 60 kg, give a large (325 mg) suppository
LARYNGO-TRACHEOPLASTIES:
Dr. Matt frequently performs laryngo-tracheoplasties for subglottic stenosis. The
stenosis is usually above the level of an existing tracheotomy. Anesthetic considerations
include the following:
Airway: The airway will be maintained intraoperatively by use of an oral RAE tube of
appropriate size cut off so that it can be inserted directly into the trach stoma and
sutured in place. Late in the case, it will be replaced with a stainless steel trach
tube with an inner cannula that will adapt to an anesthesia circuit, and will be sewn
in place. At this point in time, Dr. Matt will want you to use an extra-long, sterile
anesthetic circuit handed over the drapes. We have these made up in advance, in
sterile packs. A new, clean but not sterile end-tidal CO2 tubing can be inserted,
using sterile gloves and keeping the circuit as clean as possible.
Monitoring: Do not use an esophageal scope, as bronchoscopy will be performed both
pre- and post-repair. Usually a cartilage rib graft will be obtained from the right
chest, so keep your stethoscope head on the left. An arterial line is usually
indicated.
REFERENCES:
1.
Litman R, et al: Ondansetron decreases emesis after tonsillectomy in children.
Anesth Analg 1994;78:478-81.
2. Pappas ALS, Sukani R, et al: The effect of preoperative dexamethasone on the
immediate and delayed postoperative morbidity in children undergoing
adenotonsillectomy. Anesth Analg 1998;87:57-61.
3. April MM, et al: The effect of intravenous dexamethasone in paediatric
adenotonsillectomy. Arch Otolaryng Head and Neck Surg 1996;122:117-120.
4. Splinter WM, Roberts DJ: Dexamethasone decreases vomiting by children after
tonsillectomy. Anesth Analg 1996;83:913-6.
5. Hall S: Tonsillectomies, ketorolac, and the march of progress (editorial). Can J
Anaesth 1996;43:544-8.
6. Holt R, et al: Tropisetron plus dexamethasone is more effective than tropisetron
alone for the prevention of postoperative nausea and vomiting in children undergoing
tonsillectomy. Paediatric Anaesthesia 2000;10:181-188. (tropisetron is a 5-HT3
blocker)
7. Henzi I, et al: Dexamethasone for the prevention of postoperative nausea and
vomiting: a quantitative systematic review. Anesth Analg 2000;90:186-94
8. Goll V, et al: Ondansetron is no more effective than supplemental Intraoperative
oxygen for prevention of postoperative nausea and vomiting. Anesth Analg
2001;92:112-7
9. Wilson K, et al: Can assessment for obstructive sleep apnea help predict
postadenotonsillectomy respiratory complications? Anesthesiology 2002;96:313-22.
10. Elhakim M, Ali NM, et al: Dexamethasone reduces postoperative vomiting and pain
after pediatric tonsillectomy. Can J Anesth 2003;50:392-97.
11. O’Flaherty JE, Lin CX: Does Ketamine or magnesium affect posttonsillectomy pain in
children? Paediatric Anaesthesia 13;2003:413-421.
12. Brown KA, et al: Recurrent hypoxemia in children is associated with increased
analgesic sensitivity to opiates. Anesthesiology 2006;105:665-9.
12/06
TWolfe
LASER EXCISION of PAPILLOMAS
INTRODUCTION: We see many children for repeated CO2 laser excision of papillomas of the
upper airway. Some are new patients, but most are repeat patients that we see
periodically, some as often as monthly. Not only is the current status of their airway
important, but their old track record as well, so be sure to review the most recent
anesthetic records. Also, do your best to handle these children with care, since we will
see most of these patients over and over again.
AIRWAY MANAGEMENT: A fair percentage of these children will have a potentially difficult
airway due to scarring and papillomas. Whenever suspicion of a difficult airway exists,
an inhalation induction is prudent. Never paralyze a papilloma patient with an uncertain
airway. Once the patient has been breathed down, you can spray the cords with lidocaine
(limit 4mg/kg of 4% lidocaine, or 0.1 ml of the 4% solution per kg) before the surgeon
inserts his bronchoscope. Intubation is usually unnecessary prior to the bronchoscopy.
SPECIAL LASER CONSIDERATIONS: Protect the patient's eyes with saline-soaked eye pads, and
protect your own eyes with plastic glasses. If an ETT is in the field, it must be a
special laser-resistant tube, or be wrapped with metallic tape starting from the bottom of
the ETT just above the cuff and ending outside the mouth. The cuff should be inflated
with saline to act as a built-in fire extinguisher. Use a potent inhaled agent in air or
in up to 30% oxygen (not O2 or N2O) during lasing. Remember that N2O supports combustion
as well as oxygen.
APNEIC OXYGENATION: Often the ETT will be removed altogether for good laser access to the
papillomas. Prior to this period of apneic oxygenation, the patient should be on 100%
oxygen for at least 2 minutes. The ETT is then withdrawn through the surgeon's endoscope
and the papillomas are excised with the laser until the oximeter readings start to drop.
When this occurs, typically after 2-3 minutes, the ETT is reinserted by the surgeon
through his laryngoscope, the patient is well ventilated with 100% oxygen, and the cycle
is repeated as many times as necessary. While paralysis is not generally necessary,
occasionally a muscle relaxant may facilitate lasing by keeping the vocal cords perfectly
immobile during the excision.
SPONTANEOUS BREATHING THROUGH SUBGLOTTOSCOPE: A second method of providing a safe laser
field (ie, one with no ETT to catch on fire) is to use a subglottoscope for surgical
exposure. In this technique, after the child is induced and breathed down deeply, the
surgeon insert a subglottoscope that extends through the vocal cords, thus preventing
laryngospasm. Topical lidocaine and/or IV succinylcholine are sometimes useful adjuncts
prior to placement of the scope. Then anesthetic gases are delivered by insufflation to
the patient, who is spontaneously breathing, via the left-sided channel of the
subglottoscope. The usual gas mixture is oxygen and a high concentration of sevoflurane.
The pop-off valve on the Jackson Rees must be closed and the gas flow set to about 4-5
liters/minute to eliminate room-air dilution of the anesthetic gases. If required,
positive pressure ventilation may be provided by occluding the proximal end of the
subglottoscope with a thumb while the anesthesiologist squeezes the breathing bag. (ref
1)
EMERGENCE: During emergence, leave the child unstimulated and mechanically ventilated
until spontaneous movement occurs. Do not stimulate with a suction catheter, jaw thrust,
etc. It is very important that the child is very awake (eyes open, moving all four
spontaneously) prior to extubation, not just aroused by inappropriate stimulation. Be
prepared to wait -- a deep inhalation anesthetic of this type may require 10+ minutes for
recovery. It is desirable to extubate these patients in the OR (vs recovery room) since
it is far easier to regain airway access, if necessary, in the OR environment.
PREVENTION/TREATMENT OF POST-OP CROUP: For children with stridor preoperatively or
children who have subglottic involvement, consider giving Decadron (1 mg/kg up to 20 mg
maximum) before the airway is instrumented. For post-operative stridor, consider
nebulized racemic epinephrine (0.5 ml in 2.5 ml) in the recovery room.
GENERAL LASER CONSIDERATIONS (ref 2)
Laser
Argon
KTP (frequencydoubled YAG)
Dye
Nd:YAG
CO2
Helium-neon
Lasers Commonly Used in the Operating Room
Wavelength (nm)
General Considerations
488 to 515 (blue/green) Absorbed by hb, melanin, similar pigments. Transmitted
through clear substances. Penetration: 0.5 to 2 mm.
532 (green)
Strongly absorbed by hb, melanin, similar. Transmitted through
clear substances. Penetration 0.5 to 2 mm.
Variable with dyes
Wavelength tuned, eg 585 nm (yellow) for hb absorption and
630 nm (red) for photodynamic therapy.
1064 (near infrared)
More readily absorbed by dark tissue. Transmitted through clear
fluids. Penetration 2 to 6 mm.
10,600 (far infrared)
Strongly absorbed by water and thus by all tissue, pigmented or
not. Tissue penetration <0.5 mm.
633 (red)
Used as a low-power coaxial aiming beam for non-visible lasers
(CO2 & Nd:YAG). No significant tissue penetration.
REFERENCES:
1. Matt B, McCall J, Cotton R: Modified subglottoscope in the treatment of recurrent
respiratory papillomatosis. Laryngoscope 100;1022-24, 1990.
2. Gravenstein D, et al: Basic principles of optical radiation and some common
applications in anesthesia. J Clin Conit 1996;12:445-454.
2/02
TWolfe
BRONCHOSCOPY for FOREIGN BODY
INTRODUCTION: The mobile, curious child between 1 and 3 years of age is the most prone to
foreign body (FB) aspiration. Toddlers explore objects orally and lack molars to grind
hard food particles, especially nuts. Most aspirated FB's are food, most are radiolucent,
and most lodge in the right mainstem bronchus. The classic triad is cough, wheezing, and
decreased air entry. The chest film may be negative or, classically, may show air
trapping on the side of the FB. Less often, atelectasis may be seen beyond the FB.
EQUIPMENMT: For small children, the surgeon uses a rigid fiberoptic bronchoscope, since
flexible fiberoptic bronchoscopes do not have an adequate working channel for FB removal.
The telescopic lens fits down the rigid bronchoscope. There is an instrument channel for
very small forceps, but larger forceps must be passed down the main channel, necessitating
the removal of the telescopic lens during the use of these forceps. There is a side port
to hook up your Jackson-Rees system for ventilation. The smallest bronchoscopes are so
small that it is difficult to ventilate (eliminate CO2) while the telescope is in place,
though oxygenation is usually easily maintained. Therefore, the surgeon may need to
periodically withdraw his telescope from the main channel to let you ventilate more
vigorously for a minute or two to reduce the CO2.
ANESTHETIC CONSIDERATIONS:
Avoid preoperative sedatives or narcotics
Inhalation induction is preferred to avoid unnecessary use of relaxants and positive
pressure ventilation, especially if there is any risk of the FB moving, or if air
trapping is present.
An intravenous and administration of glycopyrrolate is desirable but not mandatory
preinduction
Laryngotracheal lidocaine (4 mg/kg, or 1 ml/10 kg of 4%) will reduce anesthetic
requirements
Dexamethasone (up to 1.0 mg/kg) given before instrumentation may reduce post-op
subglottic edema (croup)
Protect eyes and teeth during bronchoscope insertion
Patient should be in “sniffing” position for initial insertion of the bronchoscope
(towels behind occiput). After the bronchoscope is between the cords, a shoulder roll
may help the surgeon direct the bronchoscope into the bronchi.
Confirm proper placement of ‘scope with surgeon by asking him if he can see tracheal
rings (there are no t- racheal rings in the esophagus!)
Once the bronchoscope is through the cords, place a roll under the neck to facilitate
passage of the bronchoscope into distal bronchi. Turn the head to the left to allow
the surgeon to see down the right lung, and vice versa.
If the surgeon drops a FB in the tracheal or subglottic region, ascertain ventilation.
If the FB is in such a position that all ventilation is blocked, instruct the surgeon
to shove the FB back into the lung from whence it came before trying to get hold of it
again.
Some FB’s are too large to withdraw through the bronchoscope, so the forceps, FB and
bronchoscope will all have to be withdrawn through the cords as a unit. So the a FB
does not get hung up by the vocal cords closing around it, give a muscle relaxant
during withdrawal of the bronchoscope.
Following bronchoscopy, reintubate the patient for emergence, and delay extubation
until the patient is fully awake, so that you can easily differentiate any airway
obstruction due to subglottic edema from any obstruction due to residual anesthetic
effects. A very deep level of anesthesia is required for bronchoscopies, so emergence
may take as long as 15 minutes.
Postop croup may be reduced by dexamethasone (see above), by the use of humidified
oxygen, or by treatments with racemic epinephrine (0.5 ml in 2.5 ml).
A postop CXR is indicated to R/O PTX.
REFERENCES:
1. Wolfe TM, Rao CC:
1992;1:74-80.
Anesthesia for selected procedures.
Seminars in Pediatric Surgery.
Updated 2/99
TWolfe
CLEFT LIP and PALATE REPAIRS
PATIENTS: Incidence is approximately 1:1000 live births. Highest incidence occurs in
American Indians and Orientals, lowest in blacks. Cleft palate is usually an isolated
deformity or part of a constellation of deformities that do not form a recognizable
syndrome. Of special anesthetic interest is the cleft palate associated with the PierreRobin anomalad, which also includes micrognathia (small jaw) and macroglossia (large
tongue). Major clefts may include extension into the nose, complete separation and
protrusion of premaxilla, and complete clefting of the secondary palate. Secondary
defects involve tooth development, nasal growth, and velopharyngeal function (swallowing
and speech). Nearly all have middle ear disease due to eustachian tube abnormalities.
SURGERY: The cleft lip part of the defect is closed in infancy to help with maxilla
formation. Palateal defects are closed later, with skin, mucoperiosteal and bone flaps.
Free bone flaps (“alveolar bone grafts”) using bone from rib, ilium or skull are sometimes
necessary. Pharyngeal flaps (flaps attaching the palate to the posterior pharygeal wall)
are strongly associated with postoperative airway obstruction. Ancillary procedures such
as BMT’s or dental impressions are frequently performed at the same time as palate
repairs.
AIRWAY:
We usually use prebent oral RAE tubes, uncuffed for small children and cuffed for
children large enough to accept a 6.0 ETT (the smallest size available in a cuffed RAE
tube).
Secure oral RAE tube to the middle of the lower lip, using Mastisol. Cover the
regular tape with plastic tape to make it waterproof. Be sure it is directly midline
so as not to distort lip if cheiloplasty is being done.
Inadvertent extubation is the single biggest hazard in these cases, so always have a
spare (conventional) endotracheal tube available.
Inspissated secretions or blood is the second biggest hazard.
Be sure humidifier working. Do not turn it off even if patient is warm. Remember that
100% humidified gases at room temp (21 C) contain only 18 mg/L of water, vs 44 mg/L of
water for gases at 37 C. Therefore, the relative humidity of gases that were fully
saturated at room temp are only 40% saturated at body temp (ref 3).
Use small amount of PEEP (4 cm) to assure that secretions do not tend to flow down
around the ETT, especially when the patient resumes negative pressure ventilation
Observe ETT and ventilation pressures carefully after the surgeon positions the metal
mouth gag, which frequently compresses the ETT.
Intubation “tricks” include placing gauze in the cleft to keep your laryngoscope from
sliding into the cleft, approaching the airway from the far right side of the mouth to
give you enough room to maneuver the tube to intubate, and holding the RAE tube between
your fingers in such a way that it straightens out at the acute bend while you are
trying to pass it.
MONITORING:
Pulse oximetry, ET CO2, and peak airway pressures are key, since a dislodged, kinked
or occluded ETT is the main intraoperative misadventure in these cases.
Temperature is monitored in axilla to keep the probe out of the surgical field.
Hypothermia is not usually a problem in these patients. Instead, they tend to get
hyperthermic under the paper drapes. Turn off the warming mattress if axillary
temperature > 36.5 C, and initiate active cooling if temperature > 37.5 C.
EXTUBATION: Extubate awake, moving all four, eyes open. Be sure the throat pack has been
removed. Blood and secretions in their mouths make these patients very susceptible to
post-extubation laryngospasm. These patients can be very difficult to reintubate without
disrupting the repair. Be very conservative about administering any respiratory
depressant (eg, Stadol or narcotic) prior to extubation. In the recovery room, when they
are vigorous and active, the child may benefit from cautious sedation with butorphanol
(Stadol), but titrate the dose carefully starting at 5 to 10 ug/kg, observing the patient
carefully, since these patients are very susceptible to postoperative airway obstruction.
Some patients with marginal airways will have a tongue stitch placed by the surgeon, so
that gentle traction can help keep the airway open in an emergency.
USE OF EPINEPHRINE: Your choice of anesthetic agent in children should not be influenced
by the use of epinephrine by the surgeon. This is in contrast to adults, where
epinephrine is limited to 1.5 ug/kg with halothane, and higher doses (5 ug/kg) with other
agents. Children will tolerate 10 ug/kg of epinephrine in lidocaine, regardless of agent.
Safe doses of epinephrine in lidocaine are as follows:
Epi 1/100,000 in 1%
lidocaine = 10 ug/ml
Epi 1/200,000 in 0.5% lidocaine =
5 ug/ml
Epi 1/400,000 in 0.25% lidocaine = 2.5 ug/ml
1 ml/kg = 10 ug/kg epi, 10 mg/kg lidocaine
2 ml/kg = 10 ug/kg epi, 10 mg/kg lidocaine
4 ml/kg = 10 ug/kg epi, 10 mg/kg lidocaine
Note that at the maximal allowable doses of epinephrine, you will be exceeding the usually
quoted safe doses of lidocaine of 5 to 7 mg/kg.
DENTAL IMPRESSIONS: For any cleft lip, cleft palate or primary alveolar bone graft
patient in whom dental impressions are being obtained as part of the procedure, tape the
endotracheal tube in the midline of the lower lip.
REFERENCES:
1. Epinephrine for cleft palates. Anes 1983;58:574-76
2. Epinephrine-halothane interactions. Anes 1983;58:142-5
3. Shapiro B: Humidity and aerosol therapy, in Clinical Applications of Respiratory
Care. Year Book Medical Publishers, 1975, p165
2/02
TWolfe
PULSE DYE LASER SURGERY
Flashlamp-pumped, 585-nm pulsed dye lasers are used to erase vascular birthmarks. While
not a highly painful procedure, enough pain and anxiety are produced to warrant anesthesia
in children.
Specific anesthetic management depends to some degree on the location of the lesion. For
the majority of lesions, the patient may be left supine, and therefore anesthesia may be
administered by mask or LMA using any inhaled anesthetic or a propofol drip. For lesions
on the face or those requiring prone positioning, intubation may be necessary.
For lesions of the face, if a general anesthetic using oxygen and/or nitrous oxide as part
of your technique is anticipated, you must keep in mind the possible risk of flash fires.
Oxygen and nitrous oxide support combustion equally well. If a leak of gases occurs
around your mask while the surgeon is lasing the face, and the laser ignites, say, a
facial hair, then this small ignition can set off a flash fire that extends back to the
oxygen and/or nitrous oxide source (ie, your mask). (refs 1 & 2)
Flash fires can be avoided by keeping the additive concentration of oxygen + nitrous oxide
to below 30% or by using a method of airway control less prone to leakage than a mask (ie,
LMA or endotracheal intubation with a cuffed ETT). In addition, it is prudent to wet
eyebrows or other facial hairs close to the lasing site. The laser will, in the presence
of oxygen, ignite dry hair, but not wet hair or clear plastic airway equipment (ref 1).
Sevoflurane anesthesia may result in some degree of portwine stain fading, making it more
difficult to lase the lesion (ref 3).
References:
1. Epstein RH, et al: Oxygen leakage around the laryngeal mask airway during laser
treatment of port-wine stains in children. Anesth Analg 1994;78:486-489.
2. Lowe NJ, et al: Flashlamp dye laser fire during general anesthesia oxygenation: case
report. J Clin Laser Med and Surg, April 1990.
3. Tanaka K, et al: The influence of volatile anesthetics on portwine stain. Clinical
Therapeutics 1993;15:567-569.
2/99
TWolfe
DENTAL RESTORATIONS
Introduction:
Children who come to the OR for dental restorations generally fall into four categories:
Extensive dental work required
Uncooperative or retarded
Difficult airways that would make sedating them in dental clinic hazardous
Sick patients, especially those with congenital heart disease getting “pre-pump” dental
care
Anesthetic Requirements:
Nasal Intubation: To facilitate exposure, a nasal RAE endotracheal tube is preferred.
Nasal RAE tubes are preformed so that, when placed nasally, the ventilator end of the
ETT points back over the forehead. The anesthesia tubing is padded and secured with a
Velcro dental strap around the head.
Deep Anesthesia: The head and neck will be moved around during the procedure.
Therefore, a relatively deep level of anesthesia and/or paralysis will be required to
prevent “bucking”.
SBE Prophylaxis: Often indicated for the patients with congenital heart disease (see
section labeled SBE Prophylaxis elsewhere in this manual for drugs and doses.)
Steroids: we are currently giving dexamethadone 0.5 mg/kg (10 mg maximum) to dental
patients to reduce the swelling resulting from the dental dams and oral trauma, and as
an anti-emetic.
Technique for Nasal Intubation:
After inducing anesthesia and either ventilating by mask or by oral endotracheal tube:
Vasoconstriction: Shrink nasal mucosa with either 1/4% Neosynephrine. Do not spray
out of the plastic bottle – use the atomizers. Spraying directly from the plastic
bottle aimed downward will send a liquid stream of phenylephrine into the nasopharynx.
Positioning: Place head in the classic “sniffing” position (neck flexed forward, head
slightly extended). Turn head a few degrees toward the side of the nostril chosen for
intubation to bring the tube tip toward midline.
Lubrication: Lightly coat end of ETT with lidocaine jelly
Passage through nasal pharynx: Using gentle, constant pressure, advance the ETT along
the floor of the nasal passage into the oropharynx. Never direct the ETT upward, since
damage to the turbinates or even puncture of the cribiform plate may result. Do not
use an up-and-down or twisting motion on the ETT to try to get it to pass -- it will
either go with gentle, constant pressure or you must use the other nostril or a smaller
ETT. If you use the left nostril, initially rotate the tube “upside down”, so that the
sharp tip of the tube does not gouge the turbinate. Do not traumatize the nose for
placement a nasal tube – if necessary, the surgeons can always work around an oral
tube.
Warming the ETT has not been found to reduce epistaxis. However, telescoping the end
of the ETT in a red rubber catheter (10 French for ETT’s 5.0 and smaller, 12 French for
larger ETT’s) and using the red rubber catheter to “guide” the ETT through the nose,
shielding the stiff leading edge of the ETT from the nasal mucosa, significantly
reduced epistaxis. Once the ETT enters the oral pharynx, the red rubber catheter is
grabbed with a Magill forceps and disengaged from the ETT with an abrupt tug. (ref:
Watt S et al: Telescoping tracheal tubes into catheters minimizes epistaxis during nasotracheal intubation
in children. Anesthesiology 2007;106:238-42)
Passage through glottis: Occasionally the ETT will advance right into the trachea.
Often, however, you will expose the glottis and guide the ETT through by grasping the
ETT with Magill forceps and pushing it through. Often the ETT will begin to pass
through the cords, but then “hang up”, since in children the anterior attachment of the
cords is inferior to the posterior attachment. That is, the ETT will seem to pass
through the posterior glottis, only to "hang up" at the anterior commissure. When this
happens, rotate the tube at the nose 360 degrees as you gently advance, so that the
bevel at the tip temporarily points posteriorly, and the tube will “pop” through
easily. Never force a tube through the cords, since you can dislocate the arytenoids.
Confirm placement with end-tidal CO2 and bilateral auscultation, and palpate the
inflated cuff in the sternal notch. Check to make sure the uninflated tube leaks at 20
cm H2O or less.
Stabilize ETT by placing pad over forehead, then wrapping a Velcro strap around
patient’s head and over the ETT. Check that ears are not folded under the strap.
Monitoring:
Place temperature probe in left nares.
2/07
TWolfe
SELECTED OPHTHALMOLOGIC PROCEDURES
EXTRAOCULAR MUSCLE SURGERY: The most commonly performed operation in children (aside from
ear tubes) is eye muscle surgery for correction of strabismus. As currently performed,
with a reasonably deep level of potent inhaled agent with controlled ventilation, these
cases are usually uneventful. Paralysis or deep anesthesia toward the end of the case is
not required, since the surgery is extraocular. However, certain pitfalls await the
mentally unprepared.
Oculocardiac Reflex: The OCR is a vagally-mediated reflex that is normally manifest by
either bradycardia or escape PVC's. It is more likely under conditions of light
anesthesia or hypercarbia, and is triggered by tension on the extraocular muscles
(EOM's), especially the medius rectus muscle. It can be largely prevented by
pretreatment with IV atropine, but is not prevented by IM premedication with atropine.
It is usually attenuated by repeatedly pulling on the muscle. If it occurs
intraoperatively, the normal response should be:
Check oximeter -- bradycardia in a child is hypoxia until proven otherwise
Ask surgeon to release tension on muscle
Deepen anesthetic and check ET CO2 to be sure hypercarbia does not exist
If reflex recurs, consider small dose (0.01 mg/kg) of intravenous atropine
Accidental Extubation: As with any head and neck surgery where the airway is buried
under the drapes, head movement, pressure of the surgeon's arm, etc, may lead to an
accidental extubation or dislodgement of your LMA. Be sure that you are well prepared
mentally and physically (mask, spare ETT, laryngoscope, relaxant) to efficiency deal
with this contingency.
Succinylcholine: If the surgeon wishes to gauge the tension on the EOM's very shortly
after induction (ie, within about 10 minutes), then succinylcholine may be best avoided
in your induction sequence, in that succinycholine may cause a tonic contraction of the
EOM's that lasts 5-10 minutes. Non-depolarizers are OK. (ref 5)
Nausea and Vomiting: Likely due to the vagal stimulation of eye surgery, eye patient
are more likely to vomit postoperatively than most other patients. Antiemetics are of
limited efficacy in for treatment, but may be useful when given prophylactically.
Effective conservative treatment may include:
Hydration -- be sure that patient is well hydrated prior to removal of IV, so if
nausea persists the patient can go several hours without needing to take fluids PO.
Use muscle relaxants (eg, mivacron) that do not require reversal with
anticholinesterase drugs
Treat pain adequately -- prompt treatment of postoperative pain with butorphanol
(10-30 ug/kg) or morphine (up to 0.1 mg/kg) seems to greatly diminish the incidence
of clinically important N&V
Delay alimentation until patient is thirsty. Do not insist on PO fluids prior to
discharge (ref 9)
Do not force or encourage early ambulation (ref 9)
Prophylaxis of N&V:
Ondansetron (serotonin antagonist) 0.10 mg/kg IV. (ref 10,11)
Dexamethasone 0.5 to 1 mg/kg up to 20 m(ref 13)
Metoclopramide 0.15 mg/kg given IV at the conclusion of the surgery will prevent
N&V and/or lessen its severity (ref 4). Maximum dose of 4 mg, can be repeated
once.
Droperidol 15 to 75 ug/kg IV (max 2 mg) given immediately after induction of
anesthesia. This drug often results in undesirable long-term post-op sedation
(refs 1 & 2).
OCULINUM INJECTIONS: For certain types of nystagmus, the surgeons will inject a permanent
neuromuscular toxin (botulinum) into the eye muscles (see ref ). In order to judge the
effect of the injection, the surgeon will want you to avoid any agent that produces
neuromuscular weakening, eg neuromuscular blocking agents or potent inhaled agents. One
technique that has been used is to induce and maintain anesthesia with ketamine.
Intubation is generally not necessary, since these are short (15 minute) procedures.
Pretreat patient with atropine or glycopyrrolate (ketamine stimulates secretions)
Induce anesthesia with a combination of thiopental (2-4 mg/kg) and ketamine (2-4
mg/kg), unless the ophthalmologist will allow the use of an inhaled agent.
If necessary, augment technique with inhaled N2O, which does not depress NM function
like the potent inhaled agents (ref 6), given by nasal prongs.
ELECTRORETINOGRAMS (ERG's): ERG's involve stimulation of the retina with flashes of
light. This test must be performed in a totally dark environment, so the room will be
dark with the windows sealed, and the patient's head will be under a special hood. Stray
sources of light must be avoided (eg, pulse oximeters). Pulse oximeters can be used, but
must be covered. Have an emergency light source (ie, flashlight) readily available.
INTRAOCULAR PROCEDURES: Cataract extractions, glaucoma and retinal surgery, and other
intraocular procedures have special considerations of their own.
Motionless Field: The overriding concern is the maintenance of a motionless operative
field, either by adequate depth of anesthesia and/or by paralysis.
Smooth Emergence: A smooth emergence is facilitated by avoiding unnecessary
stimulation. Do not suction during light planes of anesthesia, do not move head and
neck, do not "let CO2 build up" above 40 mmHg. Instead, just leave patient on
ventilator, slowly wean rate, and leave patient undisturbed. Even though it is
desirable to avoid "bucking" in patients immediately after open eye surgery, do not
extubate these patients "deep", since the danger of having to emergently reintubate far
outweighs the risk of coughing on the ETT. IV lidocaine (1.5 mg/kg) may facilitate
smooth extubation, but may also delay emergence.
Glaucoma Medications: Remember that some of the glaucoma patients will be on ßblockers or other eye medications that may have systemic implications.
EXAM UNDER ANESTHESIA (EUA): Most EUA's here at Riley tend to be lengthy, and of course
you have to share the head with the ophthalmologist. Therefore, we intubate many of these
patients, or use an LMA.
EUA for Intraocular Pressure (IOP) Measurement: Accurate serial IOP’s are essential
for diagnosis and treatment of glaucoma. In most cases, the ophthalmologist would like
to measure the IOP early after induction, prior to intubation, since the stimulus of
tracheal intubation significantly increases IOP (ref 7). Accurate measurements can be
obtained just after the patient has entered the surgical stage of anesthesia following
mask induction. Measurements should not be done while the patient is still in second
stage anesthesia (ie, while the eyes are still disconjugate).
NASOLACRIMAL DUCT PROBING: Nasolacrimal probes, insertion of Quickert tubes, and other
procedures involving the nasolacrimal ducts usually require the presence of an ETT, both
due to the need for the surgeon to access the nose freely, and because the surgeon often
injects dye into the ducts, which then flows into the nose and airway.
FULL STOMACH / OPEN EYE: While older literature advocated an induction sequence that
avoided the use of succinylcholine, due to the chance of increasing intraoccular pressure
and extruding the vitreous, little or no evidence exists to support this view (ref 14).
In general, the technique you choose should be the one least likely to result in
aspiration in the trauma patient.
REFERENCES:
1. Lerman J, et al: Effect of droperidol pretreatment on postanesthetic vomiting in
children undergoing strabismus surgery. Anes 65:322-25,1986
2. Christensen S, et al: Incidence of emesis and postanesthetic recovery after
strabismus surgery in children: a comparison of droperidol and lidocaine. Anes
70:252-55,1989
3. McGoldrick K: Principles of Ophthalmic Anesthesia. J Clin Anesth 1989;1:297-312
4. Broadman L, et al: Metoclopramide reduces the incidence of vomiting following
strabismus surgery in children. Anes 1990;72:245-248
5. McGoldrick K: Considerations for pediatric eye surgery. International Anesth Clinics
1990;28:78-82
6. Neuromuscular effects of nitrous oxide, in Eger E (ed), Nitrous Oxide N2O. New York,
Elsevier, 1985,pp 177-184
7. Watcha M, et al: Effects of halothane on IOP in anesthetized children. Anesth Analg
1990;71:181-4
8. Jankovic J: Therapeutic uses of botulinum toxin. NEJM 1991;324:1186-94
9. Woods, AM, Berry FA, Carter BJ: Strabismus surgery and post-operative vomiting:
clinical observations and review of the current literature; a medical opinion.
Paediatric Anaiesthesia 1992;2:223-229
10. Sung YF, et al: Double-blind, placebo-controlled pilot study examining the
effectiveness of intravenous ondansetron in the prevention of postoperative nausea
and emesis. J Clin Anesth 1993;5:22-29
11. McKenzie R, et al: A randomized, double-blind pilot study examining the use of
intravenous ondansetron in the prevention of postoperative nausea and vomiting in
female inpatients. J Clin Anesth 1993;5:30-36
12. Sadhasivam S, et al: Prophylactic ondansetron in prevention of postoperative nausea
and vomiting following pediatric strabismus surgery: A dose-response study. Anes
2000;92:1035-1042.
13. Splinter W, et al: Low-dose ondansetron with dexamethasone more effectively
decreases vomiting after strabismus surgery in children than does high-dose
ondansetron. Anesthesiology 1998;88:72-75.
14. Vachon CA, Warner DO, Bacon DR: Succinylcholine and the open globe. Anesthesiology
99;2003:220-223.
Last update 7/03
TWolfe
SELECTED GU (GENITOURINARY) PROCEDURES
MEATOTOMY: Infants and children that are having a simple meatotomy may usually be done
under mask anesthesia. These cases generally require only a few minutes of operating
time. If needed, you can ask the surgeon to perform a penile block for postoperative pain
control.
CIRCUMCISION: The older the child, the longer and more involved these cases are likely to
be. Anesthesia is usually provided using a standard inhalation technique with intubation
or LMA, especially if you anticipate performing a caudal block for postoperative
analgesia.
Postoperative analgesia: A caudal block is appropriate for postoperative analgesia in
most of these children. A detailed section discussing relative contraindications,
informed consent, and the precise details of performing a caudal block appear in second
section of this manual, “Pain Management”. In general, the block is usually performed
at the end of the case, using 0.5 to 0.8 ml/kg of ropivicaine 0.2% with 1/200,000
epinephrine. Some staff add 1 ug/kg of clonidine to the caudal.
HYPOSPADIOUS REPAIR: These procedures generally require 2 to 3 hour, redos somewhat
longer.
Postoperative analgesia: Caudal analgesia is generally the preferred technique for
postoperative analgesia (see second section of this manual, “Pain Management”, for
details of technique). Ideally, the block will be performed at the beginning of the
case, so that it is effective at the time of emergence. Currently, we use 0.2%
ropivicaine approximately 3/4 ml/kg plus 2 ug/kg of clonidine (1 ug/kg of clonidine if
the patient will go home within 18 hours).
A word of caution: Do NOT assume that your caudal will reduce intraoperative
anesthetic requirements. “Bucking” in these patients prior to the application of the
tight circumferential penile dressing at the conclusion of the procedure results in
significant bleeding. Maintain an adequate depth of anesthesia until the special
dressing has been secured.
URETERAL REIMPLANTS: These procedures generally require 3 hours, though that may be
variable, depending on whether or not a cystoscopy will be performed, whether or not it is
unilateral or bilateral, and whether or not the child has had prior surgery.
Postoperative analgesia: Bladder spasm is a very significant problem following these
cases, and therefore good postoperative analgesia is highly desirable. Currently, we
usually perform caudal analgesia for these patients with either 2 ug/kg clonidine or
40 ug/kg of morphine added to the caudal ropivacaine. Any patient for which this is
done must be an inpatient, must be followed postoperatively by our pain service, and
must have specific consent for the caudal with clonidine or morphine documented (re:
risk of postoperative apnea). These patients will usually experience significant pain
when the caudal morphine wears off in about 12 to 14 hours, so a plan for dealing with
that pain must be in place. Also, a significant number of these patients who have had
caudal morphine will experience significant nausea and/or pruritis.
In addition to the caudal, a suggested supplementary regimen for children old enough or
mature enough to use a PCA is to start with a 1/2 strength PCA, and convert to full
strength after 12 to 15 hours.
Half-strength PCA: 10 ug/kg of MS q20 minutes, with a 4 hour lockout of 100 ug/kg.
Full-strength PCA: 20 ug/kg of MS q10 minutes, with a 4 hour lockout of 200 ug/kg.
In addition, Toradol 0.5 mg/kg is ordered IV q6h X 8 doses.
For patients too small or immature for a PCA, use Stadol 10 ug/kg prn q1h for the first
12 hours, and convert to 50 ug/kg of morphine q1h prn after 12 hours. As with the
older patients, Toradol 0.5 mg/kg is ordered q6h X 8 doses.
BLADDER EXTROPHY REPAIR IN NEONATES: In these children, formation of an abnormally large
cloacal membrane prevents midline closure of the musculoskeletal elements of the anterior
abdominal wall. Because of the lack of fusion, the rectus abdominis muscles and pubic
arch are separated. The anterior bladder wall is absent, and the posterior wall of the
bladder everts so that the urinary tract is wide open.
Surgery for these repairs tends to be extensive, particularly if the bony pelvis is
involved. Blood and third-space loss of fluids may be extensive. Therefore, two good
IV’s, in the upper extremities if possible, and an arterial line are indicated.
Postoperatively, a newborn bed with overhead warmer is brought to the operating room and
the infant is positioned supine with the legs radically elevated to take all the pressure
off the newly approximated pelvis. These infants are kept in this markedly legs elevated
position for 3 weeks postoperatively to keep the legs from abducting and externally
rotating, which would tend to dehisce the newly united symphysis pubis. Therefore, at the
end of the case, administer a moderate dose of non-depolarizing muscle relaxant before
transport to make initial control easier in the newborn unit.
11/04
TWolfe
OPEN HEART SURGERY
PATIENT EVALUATION: Most cardiac patients now present as “AM Admits”, although many are
inpatients. Almost all AM Admit patients will come to the Riley Heart Center (4th floor
right above the OR) for evaluation prior to the day of surgery. Usually a resident or
fellow is asked to go work up these patients in the afternoon. If you are assigned to a CV
case the next day, it would be best to be relieved to work up your own Heart Center
patient. However, if this is not possible, someone will be assigned to see the patient
and you should check to see if a completed work-up is in the Heart Center or in Day
Surgery before you leave in the afternoon.
If you are assigned to work-up a CV patient, either in the Heart Center or as an
inpatient, it is essential that you review and summarize the latest cardiac
catheterization and echocardiography data on your pre-op note. If the old chart or recent
data is not available, you can get it from the Cardiology office at 48906. Echocardiogram
reports can be found on the following website:
http://162.1.135.23/webpro/
On the login screen, the username is “cvriley”, and the password is “password”.
your patient’s name or MRN and click on the report you wish to view.
Enter
Lab results should also be reviewed, although they are often not available the afternoon
before in Heart Center patients.
When seeing the patient and family, be sure not to “promise” that their child will be
extubated following surgery. Although this is often possible, it is more important that
the family be prepared to see their child still intubated in the ICU, as is often the
case. Also, do not forget to obtain consent for caudal analgesia if this is applicable
(see below for caudal indications and dosing).
Blood products are ordered by the surgery staff.
Finally, be sure to order “drips” for your CV patients. There are preprinted orders
(“drip sheets”) available in the Heart Center (the nurse should have it stamped up and
ready for you) or in Day Surgery in the file cabinet. All you need to do is put your
patient’s name, MRN, and weight, the date, location needed (“ROR”), and time needed
(usually 0600 for 1st case of the day), then circle any drips you want to order and sign
at the bottom. Always order nitroglycerine, almost always order dopamine and dobutamine,
usually order epical, and sometimes order fentanyl. Rarely you will need nitroprusside
and/or esmolol, but this is typically for a coarctation patient at risk for post-op
hypertension. The drips will come with a preprinted table with the rate in ml/hr for the
normal dose range of the drug for a patient of the weight you specify. If you have any
questions about what drips to order, discuss it with staff.
ROOM SET-UP
Normal setup plus:
Phenyephrine 100mcg/cc
Epinephrine 10mcg/cc (put 1cc of boxed “cardiac epi” into a 10cc syringe and draw up
QS 10cc NS)
Have inotrope drips in the room
2 IVs and A-line
Add several stopcocks to drip line
Consider wide bore tubing on volume line (pts. >40 kg)
Hot line
Need blood available (check with circulating nurse)
Bair hugger
BIS monitor (for pts. with language cognition)
Cerebral oximeter (attach appropriate-sized probe to monitoring cables and have them
tucked under the head of the bed)
INDUCTION
Many, if not most, CV patients can be safely induced via inhalation technique with
sevoflurane, although unstable patients or those with complex lesions are best done
intravenously. ECG, oximeter, and NIBP are generally sufficient prior to induction.
LINES AND MONITORING
2 IV's and an arterial line are usually sufficient. In “re-do” sternotomy patients
(significant blood loss potential), use the largest-bore IV’s feasible with large bore
extension tubing on at least one of the IV’s. The arterial line and the second IV are
generally placed following induction. Avoid placing lines in arms that have been used
for Blalock shunts in the past. Avoid the left radial if a coarctation has been
repaired using a left subclavian flap. Avoid the right radial for Norwood I operations
(they get a right BT shunt). Never place a dorsalis pedis or posterior tibialis
arterial line in any patient having CPB. Post-CPB vasoconstriction almost always makes
these lines unreliable for some time after separation from CPB.
On complex cases have inotropic drips spiked and flushed from the outset. Plumb them
into the IV so that the volume of fluid that must be given to effect a change in the
drip rate is minimized.
The ET CO2 monitor will be inaccurate on patients with a marked R->L shunt, since
relative pulmonary blood flow will be markedly reduced. The ET CO2 will read
approximately 2.5 mmHg lower than arterial CO2 for each 10% reduction in oxygen SAT.
Therefore, if the SAT is 80%, the ET CO2 will read about 5 mmHg low (ref c).
At least two pulse oximeters should be used to provide backup. If you have a femoral
arterial line, consider placing one pulse oximeter on the ipsilateral foot. This can
function as a monitor for the perfusion of that extremity, which can be significantly
compromised by the femoral arterial line.
Monitor temperature with an esophageal stethoscope, but also place a nasopharyngeal
probe. This will be useful when it comes time to remove the esophageal so that the
cardiologist can perform TEE. Replace the esophageal after the TEE, as it is more
accurate than a NP temperature probe.
CAUDAL: Indications for caudal analgesia in CV surgery include any patient for whom there
is a reasonable possibility for extubation in the OR or shortly after arrival in the ICU.
The usual contraindications apply. There is no strict lower or upper weight limit, but
most of the very small infants (< 5 kg) are also not usually candidates for early
extubation. Also, because of technical challenges in older patients, some staff avoid
caudals in patients of adolescent age or older. The caudal must be placed before surgery
so that full hemostasis is attained in the area before the patient is heparinized. Dosing
is with morphine ONLY in normal saline (no local). Dose is 50 – 100 mcg/kg MS04 in 0.5 –
1 cc/kg total volume (max volume 15 cc). Both the morphine (Duramorph) and the saline
with which it is mixed must be preservative-free. Caudal morphine will normally provide
excellent analgesia for 6 to 24 hours postoperatively (ref 5 & 6).
POSITIONING
A shoulder roll will be placed behind the upper chest. Stabilize head such that it is
straight to facilitate venous drainage during the pump run. Gel pads for the head (“jelly
donut”) are available in the anesthesia work room. Arms are padded as needed and tucked
at the patient’s side. Put a long U-shaped Bair Hugger blanket in place before the prep
begins. This will be used after CPB, so do not hook up the hose until then.
PREOP MEDICATIONS
Cefuroxime, 25 mg/kg for all (non-allergic) patients
Aprotinin, an antifibrinolytic which decreases post-CPB blood loss, is indicated for
pre-bypass administration in nearly all redo sternotomies and many primary cases.
Dosing varies by surgeon:
Turrentine: 2.5 cc/kg bolus from us, 2.5 cc/kg bolus from perfusionist, and 2.5
cc/kg infusion from us over 2 hours.
Brown: 3.5 cc/kg bolus from us, 3.5 cc/kg bolus from perfusionist
Rodefeld: Leans toward the “Turrentine” technique (ask)
Adult “Hammersmith Protocol”: 200 cc from us, 200 cc from perfusionist, and 50
cc/hr infusion while on CPB.
Because of the potential for life-threatening anaphylactic reactions, do not begin the
aprotinin until the surgeon has placed purse string sutures for the arterial and venous
cannulae, so that if necessary CPB can be quickly initiated. Start with a 1 cc “test
dose” to check for allergic reactions. If the test dose is negative, slowly administer
the bolus (over approximately 20 minutes). Any remaining aprotinin not used for bolus
or infusion should be given to the perfusionist.
Steroids are given by the perfusionist for CPB cases (Solumedrol 30 mg/kg)
MAINTENANCE
A “high dose narcotic technique” continues to be the most commonly employed technique.
Recently this has often been accomplished with a remifentanil infusion. Expected
postoperative ventilation is by no means a contraindication to remifentanil, but in
these cases high dose fentanyl is often used instead. For these patients a fentanyl
bolus is introduced prior to sternotomy, 30 to 50 mcg/kg in divided doses, often
accompanied by an infusion of fentanyl, generally 10 mcg/kg/hour. A low dose volatile
agent may be used for amnesia, although in babies and children without language
cognition this requirement is minimal (particularly while hypothermic) (ref d).
However, isoflurane or sevoflurane may be given via the vaporizer on the pump
throughout bypass to provide amnesia and keep the perfusion pressure between 40 and 60
mmHg.
Remifentanil requirements vary with patient weight. Because of a higher Vd, neonates
and infants require a higher infusion rate per kg than do toddlers, older children, and
adolescents. A suggested dosage range is as follows:
<10 kg
0.6 to 0.8 mcg/kg/min
10 – 20 kg 0.4 to 0.6 mcg/kg/min
20 – 40 kg 0.3 to 0.4 mcg/kg/min
>40 kg
0.2 to 0.3 mcg/kg/min
Be aware that the hypothermia associated with CPB will significantly decrease the
activity of the blood esterases responsible for metabolizing remifentanil, causing a
rise in serum concentration (ref 10). Nonetheless, infusion rate is generally left
unchanged during bypass.
Midazolam (50 – 100 mcg/kg) is often given just before initiation of CPB and again at
the start of re-warming.
Muscle relaxation is generally accomplished with a cisatracurium infusion, 2 – 3
mcg/kg/min.
VENTILATION
FiO2 depends upon the type of lesion. Patients with low pulmonary blood flow generally
do best with a higher FiO2 (0.5 to 1.0). An exception is the patient with “Norwood
physiology”, in which “balanced circulation” must be achieved to maintain appropriate
pulmonary and systemic perfusion. Room air is the general rule for these patients, as
it is for patients with significant L -> R shunting and/or pulmonary overcirculation.
Consider, however, using 100% oxygen during the induction phase, regardless of lesion,
to avoid severe desaturation during the apneic interval, especially in neonates and
small infants.
Nitrous oxide is usually avoided, to reduce the risk of exacerbating any air embolus
that might occur. Do not use nitrous postpump, since micro air emboli are always
present. Leave 100% oxygen in the circuit during bypass so that you are sure to come
off bypass on 100% oxygen.
Put the lungs “down” while the surgeon saws through the sternum to reduce the risk of
sawing into the lung or heart. Do this by turning off the ventilator and opening the
APL valve all the way.
Maintain paralysis throughout bypass so that diaphragm does not "flip" during surgery.
Resume ventilation post pump when the myocardium begins to eject blood.
BYPASS
Heparin is injected into the right atrium by the surgeon prior to cannulating. Record
time and amount, and draw an ACT one minute after the heparin has been given.
During atrial cannulation the surgeon will ask for a valsalva maneuver to avoid venous
air embolus and fill the atrial lines. To facilitate this, manually hold inspiration
at about 20 - 30 cm H2 during cannulation.
The patient is on "partial" bypass when the heart-lung machine is first turned on, but
before the tapes around the SVC and IVC are pulled up. Once the tapes are snugged up,
the patient is on"complete" bypass and all the blood in the vena cavae must return to
the pump. At this time the perfusionist will state “I’m up to flow” and your
ventilator may be turned off. Let the surgeon and perfusionist know this by stating
“We’re off the lungs”.
While apneic on bypass:
Bag slightly distended (2-4 cm H2O)
Oxygen set at 100%
Humidifier off, gas analyzer off, pulse oximeter volume down all the way, IV
fluids set to “TKO”, and check the “pre-pump” urine total.
We use the "alpha-stat" approach to PaCO2 and pH management during bypass. See ref 9
to review this important concept.
To "squeeze the lungs" during bypass to empty blood out of the lungs for the surgeon,
gently squeeze the bag to create an inflating pressure of 15-20 cm H2O.
Bypass is a good time to set up any inotropic drips that might be required following
CPB. The present generation of IV pumps are easy to program, and come with a drug
library with preprogrammed infusion concentrations. Be sure that the concentration of
the drug you are giving matches that on the IV pump program.
When the surgeon tells the perfusionist to begin rewarming, 1) give midazolam, 2)
irrigate and suction the endotracheal tube, 3) have any desired inotropic drips ready
to go, and 4) check again to make sure the flow meters are set to 100% O2.
Restart ventilation when cardiac ejection becomes apparent, or when the surgeon
requests it. Make sure the surgeon and perfusionist are aware you are beginning
ventilation. Remember that your ET-CO2 will often read low for a while following
bypass due to air or microemboli in the lungs, leading to increased dead space (A&A
1987;66:690-2).
Prior to coming off bypass, the pump blood is usually hemoconcentrated via modified
ultrafiltration (MUF). MUF increases hematocrit and fibrinogen concentrations, reduces
total body water, and improves hemodynamics. Some red cells are recovered from the
pump circuit after MUF and are made available for transfusion if needed. MUF cells
contain heparin and therefore need to be covered by protamine, 1 mg/15 ml, when infused
(ref 8).
As soon as the patient is separated from CPB, turn on the Bair Hugger to avoid a sudden
plunge in the patient’s temperature when they are no longer receiving warm blood from
the bypass machine.
Protamine is given at the end of bypass to reverse the heparin. Dosage is calculated
by the perfusionist, who will provide you with the drug. The surgeon will direct you
to give half the protamine before the aortic line is removed. Once half the protamine
is in, be sure to inform the surgeon so that he can remove the pump suckers from the
field. Do not give the second half until the aortic line is removed, to avoid forming
intraaortic clots. Get an ACT three minutes after all the protamine has been given.
Bleeding post pump should be anticipated in smaller patients (< 8 kg) and treated with
platelets (10 ml/kg), followed by cryoprecipitate (10 ml/kg), which can restore
fibrinogen vWF and XIIIn with a small amount of transfused volume. FFP is much less
likely to be efficacious, in part due to the large volume that would need to be infused
to correct, especially, low fibrinogen levels (ref 7).
Always be certain blood is available when coming off bypass. While rare, significant
hemorrhage can result when the aortic cannula is removed.
TRANSFER TO ICU
Place all inotropic or vasoactive drips on a single IV pole, along with the carrier
fluid, to facilitate transfer.
Be sure that all drips are clearly labelled with:
mg/ml of drug in solution
weight of patient
a listing of ug/kg/hr vs drip rate to set on pump
Bundle and label arterial line, CVP, LAP, PA and other lines with their transducers in
blue towels to facilitate transport and to make it easier to sort things out in the
ICU.
REFERENCES:
1. Behnen FM, Tarhan S: Anesthesia for Surgical Repair of Congenital Heart Defects in
Children, in Tarhan S (ed): Cardiovascular Anesthesia and Postoperative
Care
Publishers, 1982, pp 73-180.
2. Pediatric Cardiac Anesthesia, ed Lake C Appleton-Lange, 1988.
3. Fletcher R: Gas exchange during anaesthesia and controlled
ventilation
heart disease. Pediatric Anaesthesia 1993;3:5-17.
4. Antognini J: Hypothermia eliminates isoflurane requirements at 20 C. Anesthesiology
1993;78:1152-1156.
5. Rosen KR, Rosen DA: Caudal epidural morphine for control of pain following open heart
surgery in children. Anesthesiology 1989;70:418-21.
6. Valley RD, Bailey AG: Caudal morphine for postoperative analgesia in infants and
children: A report of 138 cases. Anesth Analg 1991;72:120-124.
7. Miller BE, et al: Predicting and treating coagulopathies after cardiopulmonary bypass
in children. Anesth Analg 1997;85:1196-1202.
8. Elliott M: MUF and open heart surgery. Editorial in Paediatric Anaesthesia 1999;9:15.
9. Davies LK: Cardiopulmonary bypass in infants and children: how is it different?. J
Cardiothor and Vasc Anesth 1999;13:330-345. Excellent review.
10. Michelsen LG. Holford NH. Lu W. Hoke JF. Hug CC. Bailey JM. The pharmacokinetics of
remifentanil in patients undergoing coronary artery bypass grafting with cardiopulmonary
bypass. [Clinical Trial. Journal Article. Multicenter Study. Randomized Controlled Trial]
Anesthesia & Analgesia. 93(5):1100-5, 2001 Nov
11/04
LLatham/TWolfe/SWalker/MMazurek
CLOSED HEART SURGERY
PATENT DUCTUS ARTERIOSUS LIGATION
PATIENT EVALUATION: Most are premature infants recovering from IRDS (infant respiratory
distress syndrome) who fail to wean from the ventilator due to a L -> R shunt through the
patent ductus. In addition to prematurity and lung disease, these patients have usually
failed medical management (oxygen, digitalis, fluid restriction and diuretics,
indomethacin), or have a contraindication to closure of the ductus by indomethacin (eg,
renal failure or platelet dysfunction).
Older children with PDA's are often asymptomatic, but pulmonary hypertension must be
looked for and ruled out during your preoperative evaluation.
PHYSIOLOGY: In utero, 90% of the right ventricular output bypasses the high resistance
pulmonary circulation and flows into the aorta via the ductus. After birth, this flow
decreases and then reverses as the pulmonary vascular resistance falls. Normally, the
ductus closes physiologically by 10-15 hours of age, and closes anatomically by about 12
weeks of age due to muscular contraction and thrombosis. Muscular contraction is
influenced by many factors, prominent among them being arterial oxygen tension and
prostaglandin levels.
PATHOPHYSIOLOGY: Premature infants lack the normal musculature in the ductus that allows
it to contract in response to the increased oxygen tension and decreased PGE-1 and PGE-2
levels which occur after birth. Thus as PVR decreases, a L -> R shunt through the PDA
results. This in turn results in increased pulmonary blood flow and LV preload. Since
the neonatal heart cannot increase stroke volume in response to increased preload
(immature Frank-Starling mechanism due to poorly compliant ventricle), blood backs up into
the LA and pulmonary veins, and CHF ensues.
ANESTHETIC MANAGEMENT:
Monitoring: Intensive monitoring, including an arterial line, is usually indicated
even though these procedures tend to be brief, due to the preexisting condition of the
patient and the possible requirement for postoperative ventilatory support. Ideally,
the arterial line would be place preductally, in the right radial or a temporal artery,
to better estimate the PaO2 in the blood perfusing the retina and brain.
Ventilation: PEEP may be efficacious, in part to decrease pulmonary blood flow prior
to PDA ligation, and in part post-op to reexpand atelectatic lung. Anticipate the
possible requirement for post-op ventilation.
Fluids: These patients are often at risk for CHF, so fluids should be given somewhat
more conservatively than in other patients. A single good IV is sufficient.
Blood loss: Usually minimal, though the potential for massive blood loss is always
present should the surgeon lose control of the ductus or rip the pulmonary artery.
Ductal and PA tissues are quite fragile. Check blood in prior to incision.
Thermoregulation: Warm OR to 80 C during the preparation of patient for surgery, and
place one of the thermal warming packs under the patient, or use a Bair Hugger.
Positioning: Right lateral decubitus for a left thoracotomy.
Choice of Anesthetic: Use a narcotic-based technique if heart failure is a high risk,
or if you are certain that post-op ventilation will be required. In most cases, an
inhaled anesthetic is appropriate.
COMPLICATIONS:
massive blood loss
recurrent nerve injury
phrenic nerve injury
REFERENCES:
1. Lake CL (ed): Pediatric Cardiac Anesthesia. Norwalk, Appleton & Lange, 1988.
2. Tarhan S (ed): Cardiovascular Anesthesia and Postoperative Care. Chicago, Year Book
Medical Publishers, 1982, pp 85-88.
2/99
TWolfe
COARCTATION OF AORTA
INFANT vs ADULT COARCTATION: Infant coarctations are preductal and often involve long
segments of atretic aortic arch. Often the only blood flow to the lower half of the body
is provided via a patent ductus arteriosus. Therefore these patients may be
prostaglandin-dependent.
The post-ductal adult-type lesions are usually more discreet. They can be roughly divided
into low and high gradient lesions. Patients with low gradient lesions (gradient of 50
mmHg or less) often have little collateral flow. Therefore, distal pressures during
crossclamping may be low, with a high risk for spinal cord ischemia. Patients with high
gradient lesions (gradient 75 mm Hg or more), on the other hand, usually have developed
extensive collaterals, so distal pressures during crossclamping tend to be better. The
downside of extensive collaterals is that these vessels may be encountered as the chest is
opened, resulting in significant blood loss.
POSITIONING OF LINES: Since the left subclavian will be clamped during the repair, or
even sacrificed to be used as a flap to widen the aorta, venous and arterial lines should
be placed in the right arm. A backup IV may be placed in a lower extremity if necessary,
but drugs should not be infused through it during crossclamping.
DISTAL ARTERIAL LINE: A distal arterial line is required for the adult-type coarctations.
Either a posterior tibial or femoral line is acceptable. Since the femoral pulse will be
difficult to palpate, mapping out the vessel with a Doppler using a permanent marker is a
useful technique. Use the umbilicus as your aiming point. Use a Seldinger technique with
needle and guidewire, and start 2 finger breadths below the inguinal ligament. Distal
arterial lines are optional for infant coarct repairs. In general, the complications from
femoral line placement for infant coarcts does not warrant their use.
OTHER MONITORING: Ideally, in addition to a right radial and a femoral line, a blood
pressure cuff should be placed on the right arm and one on a leg. Two pulse oximeters are
usually used, one as a backup. Your main pulse oximeter should be on the right hand.
MISCELLANEOUS:
Patient will be positioned in full lateral position, left side up.
Use low tidal volumes during repair to reduce mediastinal motion.
MANAGEMENT DURING CROSSCLAMPING:
While the crossclamp is on, one guideline for adequate distal perfusion is a distal
mean pressure of at least 50 mm Hg. For adult-type lesions, volume-load the patient
with about 20 ml/kg of crystalloid solution prior to crossclamping. You may need to
let the proximal pressure rise to as high as 200 mmHg in older children if the distal
pressure is < 50 mm Hg. Evoked potentials will help you judge adequacy of spinal cord
perfusion.
Blood should be checked and ready when the crossclamps are removed, and a blood gas
should be obtained shortly after release to check for any accumulated acidosis.
STEROIDS: Surgeons often request Solumedrol 15 mg/kg prior to crossclamping. Dr.
Turrentine requires this routinely. It should be given early (shortly after induction)
to allow time for clinical effect.
EVOKED POTENTIALS: The adequacy of spinal cord perfusion during cross-clamping is
usually monitored by use of somatosensory evoked responses (SSEP’s). As in spinal
fusions (see that section), avoid giving a long-acting non-depolaring relaxant until
the stimulating electrodes have been placed over the posterior tibial nerve. Plan your
anesthetic technique so as to minimally interfere with the SSEP's. Details are in the
section on spinal fusions. In general, use a narcotic-based technique with a light
level of inhaled agent and/or propofol, along with a muscle relaxant infusion.
BODY TEMPERATURE: A cool body temperature is desirable during crossclamping to
decrease spinal cord metabolism. Keep the warming blanket off at the beginning of the
case. Let the temperature drift as low as 34 C before turning the warming blanket back
on. Actively cool (reduce room temp and turn warming blanket on cool) if temp >36 C
before crossclamping. Use a Bair Hugger to return the patient to normothermia after
the clamp is off.
POSTCOARCT HYPERTENSION: Hypertension commonly is manifest following adult-type
coarctation repair. The mechanism may relate to locally induced vasoconstriction of
distal vessels, which are not used to being pounded upon by a normal pulse, or may be
renally mediated through the renin-angiotensin-aldosterone system. If systolic pressures
are above 150, then initiate therapy with a direct vasodilator such as nitroprusside.
Occasional cases will require a nitroprusside drip for several days postoperatively.
Esmolol infusions are often required as well (25 – 200 mcg/kg/min). Labetolol in 0.1
mg/kg increments is also a useful adjunct in blood pressure control following coarctation
repair. Hydralazine also may be useful, given in titrated doses.
POSTOP PAIN CONTROL: Your anesthetic plan should take into account significant
postoperative pain, especially for the older patients. 70 ug/kg of caudal morphine, or an
equivalent dose of intrathecal narcotic, often works very well, especially when combined
with a PCA.
11/04
TWolfe, Swalker
BLALOCK-TAUSSIG SHUNT
PROCEDURE: Blalock-Taussig (BT) shunts refer to systemic-to-pulmonary-artery shunts
created to increase pulmonary blood flow. A subclavian artery may be anastomosed directly
to a pulmonary artery, or, more frequently, a Gortex tube graft is placed between the
subclavian artery and the pulmonary artery.
PATIENT EVALUATION: The typical patient for BT shunt is an infant with tetralogy of
Fallot (TOF), transposition of the great vessels with pulmonary stenosis, tricuspid
atresia or some other form of hypoplastic right heart syndrome, or other lesions that
result in inadequate pulmonary blood flow. Preoperatively, the degree of cyanosis,
presence of clubbing, degree of elevation of the hematocrit, echo and cardiac
catheterization data, and history of any "tet" spells, should be noted. Also note if a
previous BT shunt has been performed, and if so on which side.
PATHOPHYSIOLOGY: Patients with TOF are cyanotic due to R -> L shunting across a VSD with
concomitant RV outflow track obstruction. A BT shunt will increase pulmonary blood flow
via a L -> R shunt, and therefore improve overall oxygenation.
"Tet" spells occur in about half of patients with TOF, with a peak incidence at age 2-3
months, often resolving by 2-3 years. "Tet" spells may occur during feeding, crying or
defecation, or may be provoked by any catecholamine-related stress, such as starting an IV
on an unsedated toddler. The crucial point in understanding a “tet spasm is the
recognition the dynamic nature of the obstruction of the right venricular infundibulum.
This dynamic closure can be triggered by any B adrenergic stress, and is accentuated by
hypovolemia. As the right ventricular infundibulum contracts harder, it spasms down so
that right ventricular outflow is severely restricted. Hypoxia, hypercarbia and acidosis
develop quickly. As conditions worsen, systemic vascular resistance (SVR) falls, leading
to even further R -> L shunting. Children who have repeated "tet" spells learn to squat,
which increases SVR, to terminate the spell. Anything that either increases catecholamine
release or decreases SVR will worsen the "tet" spell.
ANESTHETIC MANAGEMENT:
Prevention of a "Tet" spasm or hypercyanotic episodes:
Premedication is usually indicated to reduce stress during beginning of case, eg, when
starting IV, which may precipitate a “tet” spell
Volume load vigorously. Give at a minimum 10 ml/kg before beginning the case.
Consider using ketamine for induction, since it tends to maintain SVR.
Give adequate anesthetic to avoid intense catecholamine release during stimulation.
Avoid vasodilatation, and be preapred to rapidly increase SVR with phenylephrine.
Treatment of a "Tet" spasm:
Oxygen
Volume 10 ml/kg 5% albumin to start
Alpha vasoconstriction with phenylephrine 5-10 ug/kg initially
Beta blockade with propranolol 0.02 mg/kg (or esmolol 0.5 mg/kg IV slowly followed by
200 ug/kg/min drip)
Positioning: While most Blalock shunts are done through a left thoracotomy, up to 25%
of TOF patients have a right sided aortic arch that may dictate a right Blalock.
Always check with surgeon before positioning.
Lines: The arterial line should not be in the arm on the side of the surgery, since
the Gortex shunt will be attached from the subclavian artery to the pulmonary artery.
Therefore, you will lose your signal when the vessel is clamped, and your line may be
inaccurate later due to the blood diverted to the lungs. Rarely, the artery may be
sacrified entirely, and joined end-to-side directly to the PA. Likewise, the IV should
not be in that arm, since infusion of vasoactive drugs into a partially devascularized
limb is hazardous. A single very good IV is usually sufficient.
Choice of Anesthetic: An induction and maintenance technique should be chosen that
maintains SVR and does not markedly depress the myocardium. Ketamine is often the drug
of choice for induction, with anesthesia maintained either with narcotics or with light
levels of inhalation agents, combined with a muscle relaxant.
Extubation: Vigorous infants can often be extubated immediately postop, since oxygen
saturations usually improve dramatically with the shunt.
COMPLICATIONS: On rare occasions, too much pulmonary blood flow will occur after a
Blalock, leading to unilateral pulmonary edema.
REFERENCES
1. Oshito S, et al:
1989;3:597
11/04
TWolfe
Correlation of BP and oxygenation in TOF. J Cardiothor Anes
PULMONARY ARTERY BANDING
PATIENT EVALUATION: The typical patient for this procedure is a small infant with a large
L -> R shunt due to a VSD or endocardial cushion defect. These infants tend to be very
ill, with perioperative mortality as high as 15-25% in some older series. Most of these
infants are on furosemide, and are very dehydrated; therefore venous line placement may be
exceptionally challenging. Not infrequently, these patients will be in such frank
congestive heart failure preoperatively that they will already be on ventilatory support.
PATHOPHYSIOLOGY:
L -> R shunt leading to CHF.
ANESTHETIC MANAGEMENT:
Positioning: supine; incision will be a small left thoracotomy
Monitoring: arterial line essential
Venous access: a single very good IV catheter
Postop ventilation: many of these patients will require postop ventilatory support
and/or prolonged intubation postop. Be very conservative regarding extubation.
Patients who were on ventilatory support preoperatively should be returned to
ventilatory support postoperatively.
SURGICAL COURSE: Pulmonary artery banding (PAB) is a procedure whereby the pulmonary
artery is mechnically constricted by the placement of a heavy silk tie around the
pulmonary artery. The tie is sized by placing a Hagar dilator (usually size 5 or 6) next
to the pulmonary artery, then tying the band snug around both the PA and the dilator, then
letting the PA reexpand by removing the dilator. Therefore, for a brief moment, all
pulmonary blood flow will be interrupted. After the PA reexpands when the dilator is
removed, the expected hemodynamic results are as follows:
rise in systemic pressure due to a reduction in the L -> R shunt
small to moderate fall in oxygen saturation due to a R -> L shunt at the ventricular
level.
If the oxygen SAT falls to unacceptable levels (dependent on the preoperative conditions
of the individual patient), then a looser band may need to be applied. The surgeon may
wish to measure RV and PA pressures if a looser band is required. Ideally, RV pressures
will be reduced to about 1/2 systemic.
COMPLICATIONS:
Hypoxemia/bradycardia due to a band that is too tight. The only effective treatment
for this is immediate surgical removal of the band. Atropine is ineffective when
bradycardia is due to hypoxia. Alpha vasoconstriction with phenylephrine, or volume
loading, may temporarily increase pulmonary blood flow and buy time until the patient
can be taken to the OR for debanding.
Continued CHF from a band that is too loose.
Eventual scarring and pulmonary artery stenosis, or as a child grows the band may be
relatively too restrictive, leading to RV failure and hypoxia.
2/99
TWolfe
CARDIAC CATHERTERIZATION
GOALS
Resuscitation and CPR skills in case of emergency.
Light general anesthesia to provide better conditions for the catheterization.
Anesthesia must provide quiet patient for the study with minimal hemodynamic and
ventilatory changes from the resting state.
Monitoring, especially during critical events such as dye injections.
PRE-CATHERTERIZATION VISIT
Evaluation should focus on cardiac history. Review echocardiograms, previous
catheterization data, oxygen SAT in room air, past cardiac surgeries. Physical exam
should particularly note presence of edema or cachexia, respiratory pattern, enlarged
liver, status of peripheral pulses, presence of clubbing, oxygen SAT on room air.
Most cardiac catheterization patients are now AM admits.
Preoperative midazolam often appropriate (0.5 mg/kg PO, maximum dose of 15 mg).
PREOPERATIVE PREPARATION
Monitor blood pressure and pulse oximetry continuously on all patients
Arms out of Xray field. If the hands are elevated above the head, take care to avoid
undue stretch on the brachial plexus by bringing the forearms forward (anterior to the
face) on pillows or beanbags.
Before beginning, be sure that you have readily available:
Anesthetic drugs and machine checklist
Intubation equipment and suction
Nasal cannula for routine oxygen supplementation
Jackson-Rees for crisis oxygenation
Resuscitation drugs -- be sure you know the proper dose
ANESTHETIC CHOICES (see ref 10)
Very small or very sick infants:
Frequently general anesthesia with a secured airway will be the safest approach for
very sick and/or very small infants. For the critically ill, a narcotic-based
technique is often appropriate, especially if post-op ventilation is anticipated.
Infants and children that are stable:
Objective: adequate anesthesia to facilitate catheterization with the child
spontaneously breathing room air -- positive pressure ventilation or oxygen
administration may alter interpretation of catheterization data.
Premedication with midazolam 0.5 mg/kg PO up to 15 mg when appropriate.
Frequently, children are induced by mask sevoflurance, Then an IV is placed, and
they are intubated and ventilated on the lowest FiO2 compatible with safe oxygen
saturation. Another approach is to induce with ketamine 0.5 to 1 mg/kg to reduce
the painful reaction to propofol (ref 17) then administer a propofol drip, usually
starting in the range of 150 - 200 ug/kg min. A nasal cannula or LMA is often used
with this technique.
Butorphanol (Stadol) in doses of 5-10 ug/kg for post-cath pain, or morphine 25 to 50
ug/kg.
MISCELLANEOUS NOTES
Ketamine or propofol anesthesia is general anesthesia or monitored anesthesia care
(MAC), not "IV sedation" or "anesthesia standby".
Do not attempt an IV more than twice without asking staff to help you. A "snake light"
(venous transilluminator) is always available in the cath lab.
Most patients in the cath lab can be divided into those with too little pulmonary blood
flow vs those with too much. The former can be particularly treacherous, and in
general respond to alpha vasoconstrictors. The latter are often prone to CHF, and
therefore may benefit more from inotropic support. See the special considerations for
patients with tetralogy of Fallot at the end of this section.
Be sure no air enters your IV tubing. Patients with R->L shunts (e.g. VSD, TOF) are at
very high risk for paradoxical (i.e., systemic side) air emboli.
Use careful aseptic technique. Cyanotic children or patients with synthetic material
implanted, such as a VSD patch or a prosthetic valve, are at high risk for SBE.
PAT or VT is occasionally triggered by catheter stimulation. PAT will usually resolve
spontaneously. If not, useful modalities include adenosine 50-200 ug/kg given IV
rapidly, phenylephrine 0.01 mg/kg, verapamil 0.15 mg/kg, edrophonium 0.1 mg/kg,
propranolol 0.02 mg/kg, or cardioversion at 2 joules/kg. VT will usually cardiovert
with 2 joules/kg.
Slow emergence or post-cath delirium occasionally occurs following the use of ketamine,
midazolam, or other drugs. First rule out hypoxia and low output states.
If you
think the emergence is delayed due to pre or intraoperative midazolam, give flumazenil,
10 ug/kg at a time up to max of 30 ug/kg. If you think that the emergence is delayed
due to ketamine, administer physostigmine (Antilerium) 30 ug/kg.
ANGIOGRAPHIC DYE
The choice of angiographic dye has important implications for the anesthesiologist.
Older dyes (eg, Hypaque-76 or Renografin-76) have very high osmolalities (in the range
of 2000 mOsm/kg) and are hypernatremic relative to plasma. In addition, some of these
dyes (eg, Renografin) are calcium-binding. The hyperosmolatity and hypernatremia are
responsible for acute myocardial depression and systemic vasodilation that often occurs
after injection of these dyes. Calcium binding can amplify the cardiac depression.
Our current choice of dye is iopamidol (Isovue-370). Iopamidol is non-ionized, sodiumfree, has a relatively low osmolality (796 mOsm/kg), and does not bind calcium. In
contrast to the older contrast agents, a slight increase in myocardial contractility
follows its injection (ref 6). Physical chemical properties of dyes are compared in
ref 1.
In cases where a fall in SVR may be particularly detrimental (eg, severe TOF), consider
prophylactic use of a small dose (2 ug/kg) of phenylephrine prior to dye injection.
Anaphylactic or anaphylactoid reactions, including bronchospasm, airway edema,
hypotension and dysrhythmias, can follow dye injection. Treatment may require
epinephrine.
Minor urticarial reactions can be treated with Benadryl 0.5-1 mg/kg.
Renal failure can follow excessive doses of any of the dyes. Also, all the dyes induce
an osmotic diuresis and may potentiate hypovolemia during or after the procedure.
BLOOD LOSS
Blood loss during cardiac cath is often seriously underestimated. Count 1/2 ml per
each SAT or blood gas, 5 ml for blood sent for nuclear, and 10-50 ml of blood loss into
the groin, depending on size of patient and difficulty of stick.
FLUIDS
For patients with restricted pulmonary blood flow (eg, TOF), use a balanced salt
solution such as D5RL, give a loading dose of 10 ml/kg or half the NPO deficit,
whichever is greater, then a maintenance rate of 4 ml/kg.
Use a balanced salt solution without glucose during any resuscitation. Avoid solutions
with free water, such as 1/3 NS or D5W.
Switch to a non-glucose-containing fluid during resuscitation.
RADIATION HAZARDS
The radiation hazards to the anesthesiologist are minimal if you stay 2 meters back
from the tube during fluroscopy (see ref 12). Much more radiation is released during
cines, so step back an extra meter. For comparison, mR/hr at "our" corner of the table
are:
mR/hr
7
51
805
Type of fluro
A-P lateral
Lateral
cineradiography
A mobile clear radiation shield is available for your use so that you do not need to
wear a lead apron all day.
To emphasize the importance of distance from the Xray tube, remember that 6 feet of air
is as protective as 9 inches of concrete or 2.5 mm of lead.
PHONE NUMBERS
Cath lab 4-2612
Cardiology office 4-8906
OXYHEMOGLOBIN DISSOCIATION
Cath data interpretation depends on oxygen saturation data. You should be able to
convert the SAT's to PO2's. By remembering 3 key points, you can remember the whole
oxyHbg dissociation curve:
pO2 where Hb 50% saturated (p50) = 27
normal mixed venous pO2 = 40, mixed venous SAT = 75%
pO2 of 60 = 90% SAT
By use of the Fick equation, shunt fractions can be approximated using the SAT data:
CO X (∆ O2 content) = O2 consumption
Systemic flow = (oxygen consumption)/ (arterial - venous oxygen content ∆)
Pulm flow = (oxygen uptake)/ (pulm vein - pulm art oxygen content ∆)
Pulm/Systemic flow ~ (Ao SAT - mixed venous SAT) / (pulm venous SAT - PA SAT)
SPECIAL CONSIDERATIONS FOR INFANTS WITH TETRALOGY OF FALLOT
Infants with TOF present many special challenges due to their lack of pulmonary blood
flow. They are prone to "Tet" spasms (collapse or infundibular spasm of the pulmonary
outflow tract potentiated by catecholamines or hypovolemia), and consequently very
difficult to resuscitate in the case of a crisis.
Prevention/Management of a "tet" spasm:
Give a loading dose of a balanced salt solution (Ringer's or NS) of not less than 10
ml/kg before beginning the case.
Give adequate anesthetic to avoid intense catecholamine release during stimulation
Avoid vasodilatation
Administer O2 by nasal cannula except as directed by cardiologist when he is obtaining
SAT data
Do not place monitors or IV on the arm on the side of a Blalock shunt
Consider administering a small dose of phenylephrine (2-5 ug/kg) prior to dye
injections
The cath lab nurses have a "Tet" pack consisting of Plasmanate, propranolol, and
phenylephrine that they send to the ward with the patient post cath
Watch for "Tet" spasms especially immediately post cath, due in part to hypovolemia
secondary to blood loss into the groin and to the diuretic effect of the injected
angiographic dyes, and also due to the intense catecholamine release when the infants
are awakened by the withdrawal of the angiographic catheters.
Treatment of a "Tet" spasm:
Oxygen
Volume 10 ml/kg Plasmanate to start
Alpha vasoconstriction with phenylephrine 5-10 ug/kg (see ref 5)
Beta blockade with propranolol 0.02 to 0.05 mg/kg up to 0.1 mg/kg or esmolol 0.5 mg/kg
CATHETER ABLATIONS
Preoperative preparation: These patients present with SVT from a re-entry mechanism
via an aberrant pathway and may or may not have classical WPW syndrome. Patients with
WPW may have associated structural defects (eg, Ebsteins anomaly) which could mandate
modification of your anesthetic technique. Most of these patients are on long term
antidysrhythmic therapy which may include digoxin, propanolol, quinidine or amiodarone.
The implications of concurrent drug therapy should be reviewed and discussed with your
staff. A good review of catheter ablations is in ref 19.
Intraoperative requirements: The cardiologist requires complete immobility for this 4+
hour procedure, since any movement can change the position of the catheters and disrupt
the procedure. Also, most anesthetic agents have at least some effect on cardiac
conduction, and therefore light levels are desirable. Most anesthetics are performed
using light levels of isoflurane or sevoflurane through an endotracheal tube, though
other techniques (propofol, eg) are acceptable (ref 11, 15, 16).
During the study, the cardiologist may want to stimulate the dysrhythmia with an
isoproterenol drip, starting at 25 nanograms/kg/min. Adenosine is available toterminate
the tachydysrhythmia should it be necessary. The dose is 50 ug/kg, given as a rapid
bolus, and increasing doses by 50 ug/kg increments may be used to a maximum of 250 ug/kg
(see ref 9). For diagnostic reasons, esmolol in a bolus of 500 ug/kg may be used. Both
adenosine and esmolol are very rapidly metabolized and of very short duration of action
when given as a bolus.
INTERVENTIONAL CARDIOLOGY
Increasingly, the anesthesiolgist must adapt to more invasive intracardiac procedures
performed on often critically ill infants in the cath lab (ref 14). Pulmonary stenosis
can be treated by balloon dilatation, as can some types of aortic stenosis and aortic
coarctation. An atrial septal defect can be created or enlarged by pulling a balloon or
knife blade through the septum. Occlusion devices can be inserted into the ductus
arteriosus, ASD’s, or even into VSD’s that involve the muscular (as opposed to the more
common membranous) ventricular septum (ref 20).
No ideal anesthetic technique exists for infants undergoing major interventional
cardiology. Any technique must take into account the cardiac anomoly, the requirements of
the cardiologist, and the age and condition of patient, in addition risk of major
complications from the procedure, such as catheter-induced heart block or ventricular
fibrillation, cerebral infarctions during attempts to dilate coarctations, and cardiac
perforations (ref 8).
In general, it is best to intubate these patients, both because of the possibility of
an untoward event, and also because the cardiologists often insert a TEE probe as part of
the procedure.
REFERENCES:
1. White P: Ketamine: Its pharmacology and therapeutic uses. Anes 1982;56:119-36
2. Morray J: Hemodynamic effects of ketamine in children with congenital heart disease.
Anesth Analg 1984;63:895-9
3. Goldberg M: Systemic reactions to IV contrast media: a guide for the
anesthesiologist. Anes 1984;60:46-56
4. Hickey P: Pulmonary and systemic hemodynamic responses to ketamine in infants with
normal and elevated PVR. Anes 1985;62:287-93
5. Shaddy RE, et al: Continuous IV infusion of phenylephrine for treatment of hypoxic
spells in tetralogy of Fallot. J Pediatr 1989;114:468-470
6. Hirshfield JW Jr: Cardiovascular effects of iodinated contrast agents. Am J
Cardiology 66:9F-16F,1990
7. Bridges ND, Perry SB, Keane JF, et al: Preoperative transcatheter closure of
congenital muscular ventricular septal defects. NEJM 1991;19:1312-1317
8. Malviya S, Burrows FA, Johnston AE, et al: Anaesthetic experience with paediatric
interventional cardiology. Can J Anaesth 1989;36:320-324
9. Litman RS, et al: Termination of supraventricular tachycardia with adenosine in a
healthy child undergoing anesthesia. Anes Analg 1991;73:665-667
10. Lebovic S, Reich DL, et al: Comparison of propofol versus ketamine for anesthesia
for pediatric patients undergoing cardiac catheterization. Anesth Analg
1992;74:490-494
11. Renwick J, et al: Cardiac eletrophysiology and conduction pathway ablation. Can J
Anaesth 1993;40:1053-64
12. Henderson KH, et al: Radiation exposure of anesthesiologists. J Clin Anesth
1994;6:37-41
13. Karl HW, Talbott GA, et al: Intraoperative administration of radiologic contrast
agents: potential neurotoxicity. Anesthesiology 1994;81:1068-1071.
14. Javorski JJ, et al: Paediatric cardiac catheterization: innovations. Can J Anaesth
1995;42:310-29.
15. Sharpe MD, et al: Propofol has no direct effect on SA node function or on normal
atrioventricular and accessory pathway conduction in WPW syndrome during
alfentanil/midazolam anesthesia. Anesthesiology 1995;82:888-895.
16. Lavoie J, et al: Effect of propofol or isoflurane anesthesia on cardiac conduction
in children undergoing radiofequency catheter ablation for tachydysrhythmias.
Anesthesiology 1995;82:884-887.
17. Tobias JD: Prevention of pain associated with the administration of propofool in
children* lidocaine vs ketamine. Am J Anesthesiol 1996;23:231-232,241.
18. Liem LB: Progress in cardiac arrythmia ablation: potential for broader application
and shorter procedure time. J Cardiothoracic and Vascular Anesth 1997;11:895-900.
19. Morady F: Radio-frequency ablation as treatment for cardiac arrhythmias. NEJM
1999;340:534-543.
20. Pihkala J, et al: Interventional cardiac catheterization. Ped Clinics NA
1999;46:441-464.
Update Jan ‘02
PREVENTION of BACTERIAL ENDOCARDITIS (SBE PROPHYLAXIS)
Cardiac Conditions Associated with Endocarditis
Prophylaxis Recommended
Prophylaxis Not Recommended
High Risk:
Complex congenital cyanotic disease
Prosthetic valve, homograft
Surgically-constructed shunts or conduits
Previous bacterial endocarditis
Moderate Risk:
Most other congenital malformations
Hypertrophic cardiomyopathy
Acquired valvular dysfunction
Mitral valve prolapse with regurgitation
Negligible Risk:
Secundum ASD
Repaired ASD, VSD, or PDA (> 6 months)
Physiologic, functional or innocent murmur
Mitral prolapse without regurgitation
Previous Kawasaki disease without regurg
Previous rheumatic valve dysfunction
Previous CABG
Pacemaker or implanted defibrillator
Procedures and the Implication for SBE Prophylaxis
Prophylaxis Recommended
Prophylaxis Not Recommended
Dental
Extraction, peridontal or endodontic
procedures
Respiratory Tract / ENT
T&A
Surgery involving respiratory mucosa
Bronchoscopy with rigid bronchoscope
Gastrointestinal Tract
Esophageal dilatation
Sclerotherapy
Endoscopic retrograde cholangiography
Biliary tract surgery
Surgery involving intestinal mucosa
Genitourinary Tract
Cystoscopy
Urethral dilatation
Prostatic surgery
Patient
Dental
Procedures not involving gums, roots, bone
Respiratory Tract / ENT
Intubation
BMT's
Flexible bronchoscopy with or without bx
Gastrointestinal Tract
Endoscopy with or without bx
Transesophageal echo (TEE)
Genitourinary Tract
Vaginal hysterectomy or C section
In uninfected tissue: urethral cath, D&C,
Therapeutic abortion, sterilization, IUD's
Circumcision
Other
Cardiac cath including angioplasties
Implant pacemaker, defibrillator, stent
Incision of surgically prepped skin
Prophylactic Regimens for GI and GU Procedures
Regimen begun 30 minutes before procedure
High-risk patients
Amp 50 mg/kg + Gent 1.5 mg/kg; repeat amp 25 mg/kg after 6 hr
High risk with allergy to
Ampicillen/Amoxicillen
Moderate risk patient
Moderate risk with
allergy to amp/amox
Vancomycin* 20 mg/kg given over 1-2 hours + Gent 1.5 mg/kg
Amoxicillen or Ampicillen 50 ug/kg
Vancomycin* 20 mg/kg over 1-2 hours
The incidence of "red man" syndrome is very high when vancomycin is given during the
administration of an anesthetic. Therefore, infuse over no less than one hour with
careful monitoring.
REFERENCES:
1. Dajani AS, Taubert KA, et al: Prevention of bacterial endocarditis: recommendations
by the American Heart Association. JAMA 1997;277:1794-1801.
2. Brook MM: Pediatric bacterial endocarditis. Ped Clinics NA 1999;46:275-287.
10/97
TWolfe
GLOSSARY OF ABBREVIATIONS USED IN CONGENITAL HEART DISEASE SURGERY
Ao:
AS:
ASD:
BTS:
CHD:
CoArc:
DILV:
DOLV:
DORV:
D-TGV:
EF:
HLHS:
HLV:
HRHS:
IHSS:
IVC:
LA:
LPA:
L-TGV:
LV:
LVH:
MPA:
MR:
MS:
PA:
PAB:
PAPVR:
PDA:
PFO:
PI:
PS:
PV:
RA:
RPA:
RV:
RVH:
SAS:
SVC:
TA:
TAPVR:
TGA:
TGV:
TOF:
VSD:
aorta.
aortic stenosis.
atrial septal defect. Classified as primum (1° ASD) or secundum (2° ASD).
Blalock-Taussig shunt.
congenital heart disease.
coarctation of the aorta (also CoA).
double inlet left ventricle.
double outlet left ventricle.
double outlet right ventricle.
dextro-transposition of the great vessels.
ejection fraction
hypoplastic left heart syndrome.
hypoplastic left ventricle.
hypoplastic right heart syndrome.
idiopathic hypertrophic subaortic stenosis.
inferior vena cava.
left atrium.
left pulmonary artery.
levo-transposition of the great vessels.
left ventricle.
left ventricular hypertrophy
main pulmonary artery.
mitral regurgitation.
mitral stenosis.
pulmonary atresia or pulmonary artery.
pulmonary artery banding (or band).
partial anomalous pulmonary venous return.
patent ductus arteriosus.
patent foramen ovale.
pulmonary insufficiency.
pulmonary (or pulmonic) stenosis.
pulmonary vein
right atrium.
right pulmonary artery.
right ventricle.
right ventricular hypertrophy
sub-aortic stenosis.
superior vena cava.
tricuspid atresia.
total anomalous pulmonary venous return.
transposition of the great arteries. Also known as…
transposition of the great vessels.
tetralogy of Fallot. (also called “Tet”).
ventricular septal defect.
SPINAL FUSIONS
PREOPERATIVE EVALUATION: Your evaluation should focus primarily on respiratory function.
Spinal fusions do not improve respiratory function, but at best stabilize it. The most
common respiratory defects in severe scoliosis are restriction of lung volumes (FRC, total
lung capacity, and especially vital capacity). V/Q abnormalities are common, with
increases in both A-aDO2 and Vd/Vt. PaO2 may be decreased, and in severe cases CO2
retention may occur. In extreme cases, pulmonary hypertension may be present. Children
with congenital or neurologic etiologies for their scoliosis are at much greater risk for
respiratory derangement than the "idiopathic" scoliosis typically seen in teenage females.
PREMEDICATION: To facilitate intraoperative hypotension and decrease overall anesthetic
requirements, premedicate with clonidine (Catapres) in healthy children over age 10. Give
approximately 5 ug/kg PO with a sip of water 1 hour preoperatively. Tablets are 0.1, 0.2,
or 0.3 mg each.
Clonidine is an alpha 2 agonist. It suppresses renin release, inhibits vasopressin
secretion, and decreases central sympathetic output, thereby decreasing MAC and
facilitating induced hypotension with less nitroprusside (see refs 3,4,5).
INTRAOPERATIVE SPINAL CORD MONITORING: SSER’s & EMG’s & MOTOR EP’s: Three modalities of
monitoring spinal cord function may be employed. Sensory (posterior spinal column)
function is monitored during every case via somatosensory evoked responses (SSER’s).
Motor function of individual motor roots at risk during placement of pedicle screws may be
monitored by electromyelograms (EMG’s). Finally, motor function of the spinal cord
(anterior columns) may be monitored by use of neurogenic motor evoked potentials (motor
EP’s).
SSER’s are monitored in every case. Since the motor response of the posterior tibial
nerve is essential to ensure correct placement of the stimulating electrodes, avoid nondepolarizing muscle relaxants, or use a very short-acting non-depolarizer such as
mivacurium, until after the electrodes have been positioned.
Two different types of SSER’s are recorded: cortical and subcortical. The cortical
responses are very sensitive to the inhaled anesthetics; isoflurane at > 0.5% or propofol
at an infusion rate of > 80 ug/kg/min may make the cortical responses impossible to
interpret. Pediatric patients, with their less mature neural pathways, are more sensitive
to the effects of anesthetics on cortical responses than adults.
The subcortical responses, on the other hand, are more resistant to the effects of
anesthesia, allowing more liberal use of inhaled anesthetics and/or propofol to augment
your anesthetic. The cervical lead is the reference for the subcortical responses;
therefore, it is very important that this lead is not displaced during patient movement.
Since the potent inhaled anesthetics and propofol infusions interfere with SSER's, a
narcotic-based anesthetic is normally chosen. Nitrous oxide and narcotics have almost no
effect on SSER’s. Therefore, a narcotic-based technique is an excellent anesthetic choice
for these cases, with isoflurane and/or propofol added as allowed by their effect on the
SSER’s to smooth out the anesthetic and to augment the induced hypotension. Small doses
of midazolam are usually acceptable, though large bolus doses interfere with SSER’s.
In addition to the SSER’s, which monitor sensory cord function, motor responses may be
monitored via EMG during the placement of the pedicle screws. If the screws are placed
too near the motor nerve, this can be detected as irritation via the EMG response.
Alternatively, an electrical stimulus can be applied to the pedicle screw, and the motor
response recorded on EMG.
Depending on the level of the cord at risk, EMG signals are
recorded from the rectus abdominus muscles and from lower limb or even perianal muscles.
Motor paralysis will interfere with the monitoring of the EMG responses, so the use of a
rapidly-reversible non-depolarizer (eg, mivacurium drip) is advisable to facilitate
monitoring.
Finally, neurogenic motor EP’s (NMEP’s) may be monitored to ensure that no motor injury
occurs to the spinal cord during rod distraction. Motor EP’s are monitored by stimulating
the cervical cord (via a pair of long needles placed down to the lamina at the C5-6 level)
and recording the response at the popliteal fossa from the posterior tibial nerve. These
responses can be recorded in the paralyzed patient, and are relatively resistant to the
effects of inhaled anesthetics (ref 11).
In a small percentage of patients, motor EP’s
cannot be recorded. (Note: transcranial motor EP’s, vs those stimulated at the cervical
cord, are very sensitive to low concentrations of the inhaled anesthetics, and to some
degree to barbiturates, propofol and benzodiazepines (ref 10 & 13).
If neurogenic motor EP’s are used along with the subcortical peak of the SSER, inhaled
agents can generally be used in limited amounts as part of your anesthetic technique.
Consult with the spinal cord monitoring specialist before using an anesthetic (eg, potent
inhaled anesthetics or high-dose propofol) that interferes with SSER’s. Younger patients
require higher stimulating voltages using inhaled agents. This compromises the ability to
use MEP monitoring in young children and may mandate the complete avoidance of inhaled
anesthetics (ref 14).
Refer questions regarding evoked potential, EMG or motor EP monitoring to Michelle Pratt,
R EEG/EPT, at 274-4974 or PB 4924.
ANESTHETIC MAINTENANCE: As mentioned above, a narcotic-based technique is dictated in the
presence of cortical SSEP monitoring. Examples of commonly used regimens:
Drug
Propofol
Remifentanil
Ketamine
Dexmeditomidate
Fentanyl
Sufentanil
Alfentanil
Loading Dose (ug/kg)
1-2 mg/kg
0.3 ug/kg (30 min)
10-20 ug/kg
3 ug/kg
250 ug/kg
Infusion Rate
80 – 150 ug/kg/min
0.1 - 0.3 ug/kg/min
1-2 mg/kg/hr
0.2 - 0.7 ug/kg/hour
5 ug/kg/hr
0.5 ug/kg/hr
100 ug/kg/hr
Stop Time (before
end of case)
45
12
30
15
90
60
60
min
min
to 60 min
min
min
min
min
If only SSEP's or SSEP’s plus NMEPs are being monitored, then a low level of inhaled agent
(< 0.5 MAC) plus paralysis can be combined with narcotics and propofol. This was often
the case in the past, but currently SSEP’s are usually combined with transcranial MEP’s.
When transcranial MEPS are being recorded, inhaled agents cannot be used at all, and a
total IV anesthetic (usually remifentanil plus propofol, possible augmented by ketamine or
dexmeditomidate) is used, along with a stable level of incomplete NMB with 2 twitches on
TOF, usually maintained with an infusion of mivacurium or cis-atracurium. While profound
block will eliminate the ability to monitor MEPs, a consistent partial block will both
prevent unwanted patient movement and will also prevent excessive motor activity that may
interfere with monitoring.
It is worth noting that MEPs may disappear for a long time, even after an inhaled
induction with sevoflurane, so an IV induction is preferred. Also, nitrous oxide will
also interfere with MEPs. After baseline MEPs have been established, it is often
permissible to use a paralytic for exposure only, then allow the block to wear off when
the surgeon gets to the critical portion of the case. NMB can usually be titrated such
that some block is maintained (2 twitches) but MEPs are still present. Intermittent does
of NMB should not be used, but instead an infusion (usually of cis-atracurium) should be
used to maintain a very consistent level of blockade. Do not induce total neuromuscular
blockade, as this might be interpreted as loss of MEP. The key is to maintain a very
consistent level of partial blockade.
Boluses of midazolam will obliterate the MEPs, so usually its use is confined to
preoperative premedication, if it is used at all. Boluses of even narcotics and propofol
can temporarily inhibit MEPs, so their administration should be limited to constant
infusions whenever possible.
BLOOD LOSS: Blood loss can range from less than a unit to more than a blood volume. In
patients with myleomeningocele, the dural veins may proliferate over the defect, resulting
in an excessive blood loss and risk of air embolism compared to patients with "idiopathic"
scoliosis. Also, patients with neuromuscular disease (eg, Duchenne’s muscular dystrophy,
cerebral palsy) are at much higher risk for large blood loss (ref 12). Blood loss can be
minimized by:
positoning patient so that belly (IVC) is free
use of induced hypotension
complete muscle relaxation
infiltration of incision site with epinephrine
isovolemic hemodilution
Isovolemic hemodilution can be accomplished by withdrawing blood prior to the beginning of
surgery, replacing the withdrawn blood with Plasmanate or with three times the withdrawn
volume of balanced salt solution. Citrated blood bags to facilitate isovolemic
hemodilation are kept in the blood refrigerator, in both 225 and 450 ml sizes. To
withdraw blood, place a heplock plug into the stopcock of your arterial line, and then
plug in the 16 gauge needle that comes attached to the blood bag into the heplock. Gently
agitate the blood during withdrawal to ensure mixing with the citrate -- we now have a
device to agitate the blood as it is collected.
Be sure to card-o-plate a label for the bags, and also stamp the requistion with the bag.
They can be stored at room temperature for up to 8 hours before reinfusion, though it is
better to refrigerate the blood if long delays are anticipated.
Isovolemic hemodilution is not likely to significantly reduce need for transfusion unless
at least several units are withdrawn. A mathematical analysis of the expected benefits
from acute hemodilution is found in reference 9. For example, in a 70 kg patient with an
initial hematocrit of 40 and a minimal safe hematocrit of 25, if 5 units of blood are
drawn off for later reinfusion, the savings will only amount to a single unit of PRBC’s.
For a review of the physiologic effects of isovolemic hemodilution, see ref 8.
PATIENT POSITIONING: One of the most challenging parts of the case is correct
positioning. Patients are induced on the cart, then rolled onto the OR table after the
IV's, arterial line and Foley are in place. Before you roll the patient, disconnect all
IV's and monitors so that the patient is totally free to move. As soon as the patient has
been rolled prone, immediately take a blood pressure to be sure that no orthostatic
hypotension has occurred. These patients will be in the same position for many hours, and
in addition the induced hypotension will increase the chances of regional ischemia, so be
very sure that the patient is well positioned with no unpadded pressure points.
INDUCED HYPOTENSION: Mean blood pressure is lowered to 60-65 mm Hg to provide a better
surgical field and to reduce blood loss. Be sure that the transducer is properly zeroed
at the level of the spinal cord. Either nitroprusside or nitroglycerin may be used. If
marked tachycardia occurs, consider propranolol in 0.01 mg/kg increments, or labetalol 5
mg increments, or an esmolol infusion of 200-300 ug/kg/min. For an esmolol drip, mix 2.5
gm/250 ml (10,000 ug/ml); 300 ug/kg/min of esmolol = (pt weight) X 1.8 ml/hr. Premixed
drips can be obtained from the pharmacy with dosing information attached.
Tee the vasodilator directly into your IV catheter so that no drug is left in your IV
tubing when the drip is turned off, and so that changes in drip rate are immediately
effective:
AIR EMBOLI: To reduce the risk of air emboli, keep the patient paralyzed so that the
patient cannot lower intrathoracic pressure by trying to breath. Also, keep the CVP up
(ie, keep patient well hydrated) so that venous pressure does not fall to levels that
would make air emboli more likely.
PNEUMOTHORAX / HEMOTHORAX: While using heavy instruments to decorticate the spine, an
instrument can slip far enough between the ribs to puncture the lung or lacerate an
intercostal artery.
TEMPERATURE CONTROL: For protection of the spinal cord, slight hypothermia (to 35 C) is
desirable. For temperatures below 35 C, body temperature can be easily maintained by the
application of a Bear Hugger blanket over the lower extremities. If you anticipate using
the Bear Hugger, the blanket must be put over the patient’s legs prior to draping and
prepping.
POSTOPERATIVE MANAGEMENT:
The movement of the patient from the frame to the bed must be done with care. A
coughing, bucking patient could conceivably dislodge the rod.
Naloxone has no place in the management of these patients.
Most of the patients will require a short period of ventilatory support in the recovery
room prior to extubation. A few patients, of course, will require longterm ventilatory
support. If your patient clearly falls in the latter category, then direct transfer
from the OR to the ICU is in order.
All of these patients develop a postoperative ileus, so an N/G tube should be anchored
at the beginning of the case and left in postoperatively.
POSTOPERATIVE ANALGESIA:
Most patients will be candidates for PCA’s. See section on pain management.
A method of analgesia rapidly gaining favor is the subarachnoid injection of morphine
by the anesthesiologist or surgeon at the end of the case, prior to closing. Duramorph
(preservative-free morphine), 10 to 20 ug/kg, should be injected at as low a spinal
interspace as possible. In even the largest patient, the dose of intrathecal morphine
should not exceed 1 mg to avoid respiratory depression, intractable itching and urinary
retention. Usually the surgeon can inject the morphine directly through the surgical
field, unless the fusion is too high. (ref 7)
Currently (2006) most spinal fusions are being managed with “pain balls” filled with 1%
lidocaine, plus dilaudid PCA’s postop. The pain service team has the particulars.
REFERENCES:
1. Kafer E: Respiratory and cardiovascular functions in scoliosis and the principles of
anesthetic management. Anes 1980;52:339-51
2. Yaster M, et al: A comparison of nitroglycerin and nitroprusside for inducing
hypotension in children: A double-blind study. Anes 1986;65:175-79.
3. Ghignone M, et al: Anesthesia and hypertension: The effect of clonidine on
perioperative hemodynamics and isoflurane requirements. Anes 1987;67:3-10.
4. Flacke J, et al: Reduced narcotic requirement by clonidine with improved hemodynamic
and adrenergic stability in patients undergoing cardiopulmonary bypass. Anes 1987;67:1119
5. Bloor B, et al: Reduction in halothane anesthetic requirement by clonidine, an alphaadrenergic agonist. Anesth Analg 1982;61:741-5.
6. Salem, Klowden: Anesthesia for pediatric orthopedic surgery, in Pediatric Anesthesia,
2nd ed (ed G Gregory), Churchill Livingston, New York, 1989, pp 1204-1235 (EXCELLENT
REVIEW)
7. Broadman LM, et al: Intraoperative subarachnoid morphine for postoperative pain
control following Harrington rod instrumentation in children. Can Anaesth Soc J S96.
8. Spahn DR, Leone BJ, et al: Cardiovascular and coronary physiology of acute isovolemic
hemodilution: a review of nonoxygen-carrying and oxygen-carrying solutions. Anesth Analg
1994;78:1000-1021.
9. Feldman JM, et al: Maximum blood savings by acute normovolemic hemodilution. Anesth
Analg 1995;80:108-113.
10. Ubags LH, Kalkman CJ, et al: The use of a circumferential cathode improves amplitude
of intraoperative electrical transcranial myogenic motor evoked responses. Anesth Analg
1996;82:1011-1014.
11. Bernard J, Pereon Y, et al: Effects of isoflurane and desflurane on neurogenic
motor- and somatosensory-evoked potential monitoring for scoliosis surgery.
Anesthesiology 1996;85:1013-9.
12. Edler A, et al: Blood loss during posterior spinal fusion surgery in patients with
neuromuscular disease: is there an increased risk? Paediatric Anaesthesia 2003;13:818822.
13. Sloan TB, Heyer EJ: Anesthesia for Intraoperative monitoring of the spinal cord. J
of Clinical Neurophysiology 2002;19:430-443. Best source for MEPs. Highly recommended
reading.
14. Lieberman JA, et al: The effect of age on motor evoked potentials in children under
propofol/isoflurane anesthesia. Anesth Analg 2006;103:316-321.
Updated 8/06
TWolfe
EVOKED POTENTIAL QUICK REFERENCE GUIDE
for ANESTHETIC CORRELATION
VOLATILE INHALED
AGENT
NEUROMUSCULAR
BLOCKADE
PROPOFOL
CORTICAL SSEP
(Cortically generated
response via peripheral
electrical stimulation)
HIGHLY AFFECTED
INCREASED LATENCY
DECREASED
AMPLITUDE
UNAFFECTED
AFFECTED WITH HIGHER
DOSES (ESPECIALLY IN
CHILDREN)
SUBCORTICAL SSEP
(Brainstem generated
response via peripheral
electrical stimulation)
UNAFFECTED
**AFFECTED ( WITH
INCREASED MUSCLE
TONE - NOISY
RECORDING)
UNAFFECTED
PERIPHERAL SSEP
(Recorded from the
peripheral nerve itself.
Ensures stimulus
integrity)
NMEP
(Anterior cord-motor &
possible posterior cordsensory information)
UNAFFECTED
AFFECTED (WITH
INCREASED MUSCLE
TONE - NOISY
RECORDING)
UNAFFECTED
UNAFFECTED
MUST HAVE ZERO
TWITCHES (NEED
DEEP NMB)
UNAFFECTED
EMG
(Real-time muscle
activity)
SOME ATTENUATION
AT INCREASED %
MUST HAVE 4
TWITCHES (NMB
MUST BE OFF)
UNAFFECTED
BSER
(Brainstem generated
response via auditory
click stimulation)
UNAFFECTED
UNAFFECTED
UNAFFECTED
VER
(Recorded from cortex
via visual stimulation)
The most variable EP
with use of anesthesia.
MEPs
Transcranially elicited
motor evoked potentials
HIGHLY AFFECTED
UNAFFECTED
NO DATA TO SUPPORT
MOST LIKELY AFFECTED
SINCE IT IS CORTICALLY
GENERATED
VERY HIGHLY
EFFECTED
Markedly affected
** A neck reference is used to record the subcortical component. Therefore, if there is
increased muscle tone secondary to lack of adequate neuromuscular blockade, the
subcortical component will be difficult to record and interpret.
7/04
TWolfe
VENTRICULOPERITONEAL SHUNTS
DEGREE of ELEVATION of INTRACRANIAL PRESSURE (ICP): Patients for placement of
ventriculoperitoneal shunt (VPS) can be divided into three general groups depending on
their degree of increased ICP:
MARKEDLY INCREASED: Patients with markedly increased ICP (presence of nausea and
vomiting, headaches asoociated with lethargy, papilledema, hypertension, bradycardia)
should be handled with all the normal considerations as patients with space-occupying
intracranial masses. Specifically, they should be induced with a rapid sequence
technique using thiopental (with preoxygenation and cricoid pressure) and a relaxant.
A narcotic-based technique is indicated, and the patient should be kept hyperventilated
to a PaCO2 of 25 until the ICP has been surgically relieved. When the surgeon enters
the peritoneal cavity, bucking may occur, increasing ICP. Therefore, relaxation should
be maintained during this part of the procedure.
MODERATELY INCREASED: Following an induction sequence similar to that described above
for children with more markedly increased ICP, anesthesia can be maintained with an
inhaled agent once hyperventilation has been established. A mild degree of
hyperventilation should be maintained until the shunt is established and the ICP
reduced. In general, the surgeons would prefer a technique that allows for rapid
emergence for neurologic evaluation, so avoid large doses of drugs with sedative
effects, such as butorphanol (Stadol) or midazolam.
MINIMALLY INCREASED: In these patients, such as the patient for elective shunt
revision, more flexibility can generally be exercised. An inhalation induction with
sevoflurane, for instance, is acceptable. Again a light inhalation technique combined
with a non-depolarizing relaxant is usually appropriate.
PRETERM INFANTS: Often premature infants present for VP shunts, often following an
intraventricular hemorrhage. These infants are at particular risk for postoperative apnea
and bradycardia, and should therefore not be extubated at the end of the case even if they
appear to be awake and breathing. Unless they are extremely vigorous and totally awake,
take them to recovery room intubated and monitored with a pulse oximeter. If
postoperative ventilation is required, they can normally be taken directly back to the
newborn ICU without going to the recovery room, since the period to extubation is very
variable. If you do take the infant straight back to newborn, be sure that you turn the
patient over to a responsible, knowledgeable physician. Inform him fully regarding
fluids, relaxants, and narcotics given during the case. Many of these tiny infants are at
risk for opening a ductal shunt, so be conservative in the volume of IV fluids that you
administer.
Interestingly, in preterm infants with open fontanels, ICP actually drops with induction
of anesthesia with isoflurane, halothane, fentanyl (20 ug/kg) or even ketamine (2 mg/kg).
See ref 1. Fortunately, preterm infants have a "pop-off" in terms of their open
fontanels, so sudden marked increases in ICP are less likely than in older patients.
PREMEDICATION: Patients requiring VP shunts by definition are at risk for increased
intracranial pressure. In addition, many of these patients are particularly prone to
develop postoperative apnea once the shunt has been placed. Therefore any premedicant
likely to increase PaCO2 preoperatively or likely to delay emergence postoperatively
should be avoided.
POSITIONING: Shunt patients are generally positioned supine, head turned to the left,
with a moderately large roll beneath the shoulders. The shunt will be placed on right
side of the head, above and behind the ear. The shunt is then burrowed subcutaneously
down the neck and across the chest, and the end inserted into the peritoneal cavity. An
occasional patient will have left sided or even bilateral shunt placement. The anesthesia
machine will be down the left side of the table, with the head of the patient positioned
in the center of the room. Bring your endotracheal tube out the left corner of the mouth
so that your tape with not encroach on the operating field.
EXTUBATION: All VP shunt patients, not just the prematures, are at increased risk for
apnea, airway obstruction, and aspiration postoperatively. Delay extubation until the
patient is fully awake, moving all four.
If in doubt, take to recovery room intubated.
STERILITY: The most common devestating complication of VP shunt placement is subsequent
infection of the shunt. To help reduce the infection rate, room traffic should be
minimized (ref 3,4). Only your staff should enter the room while the procedure is in
progress.
RECOVERY AND POSTOP: Postoperative apnea is the most common serious complication seen in
the recovery room. While rare, you must:supine, head turned to the left,pidural,
subarachinoid or intraventricular hemorrhage in the recovery room. Postop hemorrhage
presents with signs of increased intracranial pressure, including severe headache,
vominting, lethargy, focal neurologic signs, and neurogenic pulmonary edema (ref 5).
REFERENCES
1. Friesen et al: Changes in fontanel pressure in preterm infants. Anesth Analg
1987;66:431-4.
2. Eintrei C, et al: Local application of Xenon 133 formeasurement of regional CBF
during halothane, enflurane, and isoflurane anesthesia in humans.
Anes 1985;63:391-4.
3. Choux M, et al: Shunt implantation: reducing the rate of shunt infection. J
Neurosurg 77:875-80, 1992.
4. Duhaime A, et al: Distribution of bacteria in the operating room environment and its
relation to VP shunt infections: a prospective study. Child’s Nerv Syst 7:211-14, 1991.
5. Rose J: Neurogenic pulmonary oedema complicating ventriculoperitoneal shunt revision
in a child. Paediatric Anaesthesia 1994;4:259-62.
Updated
2/99
TWolfe
MYELOMENINGOCELE CLOSURE
INTRODUCTION: Myelomeningoceles (MM’s) usually occur in the lumbar region, though may
occur at any level. The exposed CNS tissue presents a threat of infection, so surgical
closure of the defect should be done within the first 24-36 hours of life. The spinal
cord is tethered caudally by the sacral roots, which results in orthopedic and neurologic
problems later in childhood. An Arnold-Chiari malformation is always associated with MM,
so most infants with MM will require a ventriculoperitoneal shunt within a few days of
their MM repair.
Components of the Arnold-Chiari malformation:
1. Downward displacement of the inferior cerebellar vermis into the upper cervical
canal.
2. Vagus nerve involvement, including the recurrent laryngeal nerve, which can
result in stridor and/or recurrent aspiration
3. Glossopharyngeal (IX) involvement, which innervates the carotid bodies, resulting
in loss of hypoxic ventilatory drive
ANESTHETIC CONSIDERATIONS:
ICP is usually not elevated at the time of the MM repair.
All of these infants are only a few hours of age, and have not yet been thoroughly
evaluated. They often have other defects, particularly heart defects, that have not
yet been diagnosed.
Pulmonary function is typical of the newborn: pulmonary hypertension and a tendency to
shunt rightⲺto-left through a ductus arteriosus or foramen ovale.
Positioning: For intubation, the infant is placed supine on a sterile blue towel, on a
piece of blue foam that has a hole cut out to correspond to the MM defect, so that the
infant can be place briefly supine for intubation without putting pressure on the
exposed neural tissues. For the surgical procedure, the infant is turned prone and
placed on chest and hip rolls, allowing the belly to hand free, both to facilitate
ventilation and, more importantly, to decrease intraabdominal pressure and venous
distension.
Induction technique is usually not critical. Maintenance is normally with an inhaled
agent.
Blood transfusion is rarely needed except in those cases where the surgeon must perform
a kyphectomy in order to close the defect. When a kyphectomy is required, blood loss
may be very substantial.
Monitoring: consideration should be given to placement of an arterial line, since
these are lengthy cases done in the prone position on newborns who may have other as
yet undiagnosed defects, including major heart defects. If a kyphectomy is
anticipated, the case for arterial line monitoring is very strong.
The most critical time perioperatively is the immediate postoperative period. These
infants are very prone to periods of apnea. Analgesics or sedatives should be used
very judiciously if at all. Often postoperative analgesics are unnecessary, since the
infant may be insensate at the level of the surgery. An occasional infant will require
postoperative mechanical ventilation.
As with all newborns, careful attention must be given to maintenance of body
temperature, especially during the phase of induction and placement of lines, when the
infant is fully exposed.
7/02
TWolfe
CRANIECTOMY
INTRODUCTION: Most infants presenting for craniectomy have sutures that have closed
prematurely (craniosynostosis). Typically the child will be 3 to 6 months of age. A few
will also have other congenital anomalies (eg, Apert's, Crouzon's or Pfeiffer's
syndromes), but most craniectomy patients are otherwise healthy infants.
Blood loss is by far the most important anesthetic consideration, for the following
reasons:
The usual range of blood loss in these patients is between 0.5 and 1.5 blood volumes.
Most will require transfusion.
Many of these infants are done at the age (3 months) when the "physiologic" anemia is
of infants is most pronounced.
Any blood loss that lowers central venous pressure (CVP) will put these infants at very
significant risk of venous air emboli.
Infants are particularly susceptible to hypocalcemia with the infusion of citrated
blood products, such as fresh frozen plasma (FFP) or platelets. Packed RBC's have only
a minimal amount of citrate.
ANESTHETIC CONSIDERATIONS
Blood loss:
Vascular access usually consists of two IV's and an arterial line.
Liberally replace any NPO deficits with a balanced salt solution (BSS) usually
loading with between 15 and 25 ml/kg. Either Ringer's lactate (RL) or Plasmalyte A
may be used as your BSS. Plasmalyte A has the advantage of slightly higher
osmolality.
Continue BSS throughout the case, usually at a rate of 10-15 ml/kg/hr total, since
large amounts of fluids are lost to "third space" in the scalp wound. Avoid
excessive glucose by keeping the amount solution containing 5% dextrose (ie, D5RL)
to < 5 ml/kg/hr.
Be prepared to begin transfusion with incision. Some staff elect to begin
transfusion with incision and aim to stay 5-10% ahead of blood losses, while others
volume load early with crystalloid.
Monitor ionized calcium. While little calcium is required to cover packed RBC's,
which contain little citrate, FFP or platelets will require at least 1-2 mg calcium
per ml infused to avoid hypocalcemia. Aim to keep the ionized calcium at
approximately 5.0.
Monitor volume status with continuous pressure via arterial line, base deficit, and
urinary output. Urinary output should be between 1-2 ml/kg/hr.
Positioning:
Many of these patients will be prone, necessitating special care in securing your
endotracheal tube, and in protecting the eyes.
Do not position head above heart, since that will increase the risk of air emboli.
Air emboli: As mentioned, these patients are at particular risk for intraoperative air
emboli. Precordial Doppler probes detect some degree of embolus in a large percentage of
patients (ref 1 & 2). Management should include:
a. Monitor precordial Doppler (4th right interspace) and end-tidal CO2 and N2.
Urge surgeon to not position head any higher above heart than necessary.
Keep CVP up with liberal fluid and blood replacement.
In case of embolus:
N2O off
have surgeon flood field and/or staple wound closed
If CVP in place, attempt to aspirate air
position head down (little evidence of efficacy, however)
infuse volume rapidly
consider alpha vasopressor (5 to 10 ug/kg phenylephrine)
CPR (can be done in prone position if necessary)
Emergence and Recovery:
Otherwise healthy patients may be extubated at the end of the case.
Do not over-sedate in the recovery room. These patients do not have a large
requirement for analgesics, and the neurosurgeons prefer these patients to be awake
and reactive postoperatively.
Craniectomy patients go the ICU postoperatively. Occasionally, our ICU service will
be asked to follow overnight, but in most cases Neurosurgery will manage the
patient.
References:
1. Faberowski LW, et al: Incidence of venous air embolism during craniectomy for
craniosynostosis repair. Anesthesiology 2000;92:20-3.
2. Mirski MA, et al: Diagnosis and treatment of vascular air embolism. Anesthesiology
2006;106:164-77.
1/07
TW
PECTUS EXCAVATUM REPAIR
INTRODUCTION: Most pectus deformities, especially those in adolescent males, are done for
purely cosmetic considerations. Up to 30% of patients with a pectus deformity may have a
connective tissue disorder, particularly Marfan syndrome1. Therefore, preoperative
evaluation should focus on eliciting evidence of mitral valve prolapse, aortic aneurysm,
or scoliosis. While an occasional patient will complain of shortness of breath with
exertion, pulmonary function tests are almost always normal. Due to the geometry of the
chest, many of these patients will have innocent systolic ejection murmurs.
SURGICAL PROCEDURE: In the past, the surgical repair usually involved extensive
dissection of the costochondral junction, separating the ribs from the sternum. The
procedure required 3 or 4 hours, and blood loss could be substantial. In more recent
times, the repair is much less invasive, involving the insertion of a bent steel “pectus
bar” (Nus bar) under the sternum, without extensive dissection.
INTRAOPERATIVE CONSIDERATIONS: The older surgical procedure required extensive monitoring
(arterial line) and very good IV access due to possible blood loss. The newer procedure
is much less invasive, and an arterial line is optional, though extensive blood loss or
pneumothorax is still possible. Maintenance can be with any technique, though use of some
intraoperative narcotics may be desirable for postoperative pain management.
POSTOPERATIVE CONSIDERATIONS: With the older, more extensive repair, splinting and a
partially flail chest often resulted in the need for postoperative ventilation. That is
not the case with the newer repair. However, following the newer repair (Nus bar), the
patient has to lie nearly immobile on their back for the first 48 hours. This requires
aggressive pain management (ref 2).
Usually we provide analgesia with a thoracic epidural with ropivicaine. The catheter is
threaded via the T7, T8 or T9 interspace, depending on which one is the most open
interspace. Frequently a paramedian approach is preferred, since in older teens the
posterior thoracic interspaces are often angled steeply cephalad and narrow. The catheter
is then threaded cephalad approximately 4 to 5 cm. Following a test dose of
__________________________, the epidural is loaded with 0.5% ropivicaine plain, giving
about 0.75 ml/dermatome to block. For a pectus, you need to block about 8 dermatomes, so
a typical loading dose might be 8 X 0.75, or 6 ml, perhaps 8 ml in large patient. An
infusion of 0.3% ropivicaine is then started at about 6 ml/hr, which would be 6 X 3 = 18
mg/hr. The infusion cannot exceed 0.4 mg/kg/hr, so for a 60 kg patient the maximum
infusion rate would be 60 X 0.4 = 24 mg/hr, or 8 ml per hour of 0.3% ropivicaine.
A Dilaudid (hydromorphone) PCA can be used to supplement the epidural.
Incremetal dose 2.5 ug/kg
(150 ug for a 60 kg patient)
Lockout interval: 15 min
4 hour limit: 40 ug/kg
(2400 ug for a 60 kg patient)
Rescue dose:
5 ug/kg
(300 ug for a 60 kg patient)
After 18-24 hours, ketorolac (Tordal) can be added to the regimen at 150 ug/kg per dose,
for 6 doses q6hr. For a 60 kg patient, each dose would be 9 mg.
For muscle spasms or anxiety, diazepam (0.07 mg/kg to a maximum of 5 mg) can be added q4h.
For a 60 kg patient, this would be 4 mg q4h.
On POD 2, you might want to consider adding Tramadol 1-2 mg/kg/dose PO q6h, in increments
of 25, 50 or 100mg. For a 60 kg patient, give 100 mg q6h.
In the past, we often placed a lumbar epidural and administered epidural narcotics. A summary of epidural
catheter usage for epidural narcotics:
1.
2.
3.
4.
Place at L3-4 level at beginning of case, and thread in 3-4 cm cephalad.
Load with PF morphine, 40-50 ug/kg in NS (1/2 ml/kg, 20 ml maximum) at beginning of
case
Run infusion of morphine of 2 mcg/kg/hr, increasing to no more than 5 ug/kg/hr
Pharmacy order for PF morphine:
2 mg PF morphine/100 ml NS for children < 20 kg
5 mg PF morphine/250 ml NS for children > 20 kg
Note that 0.1 ml/hr of the above concentration = 2 ug/kg/hr.
Send the Rx for the PF morphine to the pharmacy, so that it can be started in the recovery
room. The floors cannot initiate epidural infusions.
A half-strength PCA may be used to supplement the epidural.
If an epidural is not used, a full-strength PCA is an alternative.
morphine PCA settings:
Loading: up to 0.1 mg/kg
Incremental dose: 0.025-0.04 mg/kg
Lockout interval: 10-20 minutes
4 hour limit: 0.25 mg/kg
Continuous infusion option: 0.02-0.05 mg/kg/hr
Typical full-strength
PECTUS BAR REMOVAL: The metal bar that holds the sternum out during the healing process
is removed some months after the repair. This is usually a relatively minor surgical
procedure, though it is possible to get bleeding from the great vessels when the bar is
removed. Some staff will start an arterial line for these procedures.
REFERENCES:
1. Hawks P, et al: Outcome of pectus excavatum in patients with Marfan syndrome and in
the general population. J Pediatr 1989;115:954-8.
2. berde, D: Epidural analgesia in children (editorial). Can J Anaesth 1994;41:555-60.
2/06
TWolfe
MEDIASTINAL MASSES
PATHOPHYSIOLOGY: Most mediastinal tumors in children arise from the anterior compartment,
in close proximity to the trachea and the pulmonary artery. Numerous reviews document
life-threatening airway or cardiovascular crises following the onset of positive pressure
ventilation with the induction of anesthesia. The mechanism of the crisis is pressure on
the tracheobronchial tree or on the vascular structures. With the onset of positive
pressure ventilation, air trapping can occur due to a ball-valve mechanism -- that is, as
air enters the lung under pressure, the lung pushes on the mass, which in turn pushes on
the trachea and prevents exhalation. This results in a vicious cycle. As ventilation
deteriorates, further attempt at positive pressure ventilation results in even greater
pressure of the mass on both the airways and on vascular structures, especially the great
veins and pulmonary artery, resulting in combined ventilatory and vascular collapse (ref
1,2,3).
ANESTHETIC MANAGEMENT: Several signs or symptoms alert the anesthesiologist to a high
probability of a respiratory and/or cardiovascular collapse. Dyspnea is not evident until
the airway is narrowed by at least one third (ref 4). Intolerance of the supine position
indicates that the weight of the tumor is compressing the trachea or great vessels.
Facial edema indicates superior vena cava obstruction.
Preoperative CT scans are indicated for nearly all children prior to induction of
anesthesia for biopsy or resection of a suspected anterior mediastinal mass. If airway
narrowing exceeds 50%, then shrinkage of the tumor by chemotherapy, steroids, and/or
radiation therapy is indicated if feasible preoperatively (ref 5). Pulmonary function
tests may show, especially, a prolonged expiratory phase. Echocardiography may help to
delineate any involvement of the great vessels.
If anesthesia is required in a child at high risk, anesthesia is usually induced using an
inhaled agent, with the child in his preferred (usually sitting) position. Spontaneous
ventilation should be preserved as long as possible (ref 6). If ventilation suddenly
becomes difficult or impossible, a rapid change in body position (usually from supine to
prone) may relieve pressure on the airway. Rigid bronchoscopy may splint open an
obstructed airway. Emergent thoracotomy may be required to reduce pressure on the tumor
due to air trapping. In rare cases, preparation for emergent institution of
cardiopulmonary femoral-femoral bypass may be warranted.
An algorithm for perioperative management of the child with anterior mediastinal mass is
on the next page (ref 7).
REFERENCES:
1. Ferrari LR, Bedford RF: General anesthesia prior to treatment of anterior mediastinal
masses in pediatric cancer patients. Anesthesiology 72:991-995, 1990
2. Halpen S, Chatten J, Meadows A T, et al: Anterior mediastinal masses: Anesthesia
hazards and other problems. J Pediatrics 102:407-410, 1983
3. Bray RJ, Fernandes FJ: Mediastinal tumour causing airway obstruction in anaesthetized
children. Anaesthsia 37:571-575, 1982
4. Kirks DR, Fram EK, Vock P, et al: Tracheal compression by mediastinal masses in
children: CT evaluation. AJR 141:647-651,1983
5. Shamberger RC, Holzman RS, Griscom NT, et al: CT quantitation of tracheal crosssectional area as a guide to the surgical and anesthetic management of children with
anterior mediastinal masses. J Pediatric Surg 26:138-142, 1991
6. Sibert KS, Biondi JW, Hirsch NP: Spontaneous respiration during thoacotomy in a
patient with a mediastinal mass. Anesth Analg 66:904-907,1987
7. Wolfe TM, Rao CC: Anesthesia for selected procedures. Seminars in Pediatric Surgery,
1992;1:74-80.
2/99
TWolfe
HERNIORRAPHY
Multiple anesthetic techniques are appropriate for hernia repairs. Postop analgesia can be provided by using a caudal block, by placement of an ilioinguinal
nerve block by the surgeons, or by having the surgeon instill 0.2 ml/kg of 0.5%
bupivicaine subfascially at the completion of the surgery (ref 1). All three techniques
are very effective.
References:
1. Machotta A, et al: Comparison between instillation of bupivicaine versus caudal
analgesia for postoperative analgesia following inguinal heriotomy in children.
Paediatric Anesthesia 13;2003:397-402
last updated 7/03
Twolfe
NISSEN FUNDOPLICATION
INTRODUCTION: The Nissen procedure is indicated for patients with gastroesophageal reflux
with pulmonary aspiration. Therefore, these patients are at risk for aspiration during
induction, and often have pre-existing airway disease which is frequently poorly
documented.
ANESTHETIC MANAGEMENT:
Airway Management
Consider premedication with a non-particulate antacid, H-2 blocker, or gastric
motility stimulants:
30 ml sodium citrate PO
cimetidine 7.5 mg/kg IM or PO
metaclopramide 0.15 mg/kg
Rapid sequence induction with preoxygenation and cricoid pressure may be
appropriate, though mask induction is safe in most cases
Secure ETT with extra care, since you will have to manipulate a dilator through the
mouth
Delay extubation until patients are fully awake. Occasionally Nissen-type patients
will require postoperative ventilatory support. Consider glycopyrrolate for
patients with excessive secretions prior to extubation.
Monitoring
Use a precordial stethoscope and an axillary temp probe, since a dilator will be
passed via the esophagus during the case.
An arterial line may be indicated, for one or more of the following reasons:
Presence of pre-existing pulmonary disease, often poorly documented
high upper abdominal surgery, which may compromise postoperative ventilation
surgical tendency to compress the IVC, leading to acute hypotensive episodes
occasionally a retractor will lacerate the liver or spleen, resulting in massive
hemorrhage
Relaxation: Profound neuromuscular relaxation is mandatory for this high, deep
abdominal surgery. Usually a muscle relaxant infusion (eg, cis-atracurium, 2-3 ug/kg
min) is administered to keep the response on the neuromuscular stimulator at one twitch
or less. Failure to provide adequate relaxation will result in poor surgical exposure,
and also may result in trauma to the spleen or liver if undo pressure on the retractors
is necessary.
Gastric Tube and Esophageal Dilator: At the beginning of surgery, anchor a large bore
orogastric tube (Salem type) to keep the stomach decompressed. Leave the gastric tube
vented or on low intermittent vacuum.
When the surgeon is ready to cinch down the Nissen repair over the lower esophagus, he
will have you remove the gastric tube and replace it with a large dilator, over which
he will "size" the Nissen repair. The dilator tends to back itself out, since it is
tapered. If the dilator does not stay far enough down, the surgeon will do the Nissen
around the narrow taper and the repair will be too tight.
If the surgeons do not do a gastrostomy as part of the procedure, they will want you to
gently pass an N/G tube through the distal esophagus that has been narrowed by the
repair while the belly is still open. This is so that they can confirm placement of
the N/G, which may be difficult to pass due to the now narrowed esophagus, under direct
vision. This N/G will be left in place post-op and should not be pulled out for
extubation, since it may be difficult to pass another one without traumatizing the
narrowed lower esophagus.
Laparoscopic Fundoplications: Nissen procedures are now being regularly performed via
a laparoscope with insufflation of CO2 into the abdomen (ref 1). Problems relate to
the increased intraabdominal pressure: hypotension due to reduced venous return, and
hypoxemia due to atelectasis. Hypercarbia may result from hypoventilation or secondary
to the insufflated CO2. Pneumothorax can complicate the procedure.
REFERENCES:
1. Sfez M, et al: Cardiorespiratory changes during laparoscopic fundoplication in
children. Paediatric Anaesthesia 1995;5:89-95.
Updated 2/99
TWolfe
NEONATAL SURGERY
ANOPLASTY: Infants for anoplasty as newborns must be examined for other midline defects,
especially congenital heart disease. Some will have passed meconium and not be
obstructed, but others will have imperforate anus and bowel obstruction, necessitating
full stomach precautions (usually awake intubation). Avoid paralysis with a nondepolarizing muscle relaxant if the surgeon is going to use a nerve stimulator to check
sphincter tone. Paralysis with mivacurium for intubation is acceptable, as the relaxant
will have worn off before the surgery begins.
TRACHEOESOPHAGEAL FISTULA (TEF): TEF is a relatively common congenital defect, occurring
in about 1 of 3000 live births. Several types of defect can occur, but by far the most
common is the esophageal atresia with a fistula connecting the trachea to the lower
esophagus. Diagnosis is made in the newborn by the inability to pass an orogastric
suctioning catheter.
Type C is most common (85%) with blind proximal pouch and fistula between distal trachea
and esophagus. Type A, esophageal atreasia alone, is next most common. H-type fistulas
(type E above) often present with pneumonia as the initial manifestation.
50% of TEF patients are premature or SGA (wgt < 2500 gm). Survival is good if there is no
associated anomaly, especially heart disease. 35% of TEF patients have an associated
cardiac anomaly, the more common being VSD, PDA, TOF, ASD, and coarctation of the aorta.
Musculoskeletal, gastrointestinal, and genitourinary anomalies are also frequent.
Anesthetic Considerations for Complete Primary TEF Repair
Most infants currently undergo a complete primary repair, without placement of a
preopertive g-tube. This complicates anesthetic management, in that the stomach is not
vented, and therefore can be inflated by positive pressure ventilation.
The specific anesthetic management should include antisialogogue -> placement of awake
ETT -> induction with inhaled agent maintaining spontaneous ventilation -> verification
of correct ETT placement with fiberoptic "spaghetti" scope -> spontaneous ventilation
until chest open.
Arterial line monitoring is essential, since the great vessels can easily be kinked,
resulting in sudden drops in blood pressure. Also the pulmonary and metabolic state of
these infants is unstable. Blood loss is seldom an issue, so a single very reliable IV
is acceptable. Place a stethescope over the down (left) lung to monitor ventilatory
sounds during the case.
Surgical incision is a right thoracotomy, so patient is positioned full lateral, right
side up. Right lung will be largely collapsed. Profound paralysis, initiated at the
time the chest is opened, is necessary to immobilize diaphragm.
Early in the case, the surgeon will ligate the fistula, which will make positive
pressure ventilation much easier.
To find the upper pouch for the repair, the surgeon will ask you to insert a #12 red
rubber catheter into the upper pouch.
Be sure surgeon preps far enough anterior so that emergency g-tube can be done if
necessary.
In some cases (ventilation difficult), consideration should be given to balloon
occlusion of the TEF with a 2 or 3 Fr Fogarty catheter passed through a rigid Stortz
bronchoscope (A&A 1995;81:866-9). After the TEF is occluded the bronchoscope is
removed and the patient normally intubated for the primary repair.
Leave infant intubated, ventilated and paralyzed at the end of the case to facilitate
easy transport and changeover to the neonatologist.
Considerations for Gastrostomy prior to TEF Repair:
In infants with a compromised airway, or who he severe cardiac disease, a g-tube is
often inserted to decompress the stomach and facilitate positive pressure ventilation
prior to the TEF repair.
Keep upper pouch suctioned, since infant cannot swallow secretions.
Avoid positive pressure ventilation (inflate stomach -> reflux of gastric contents
through fistula into lungs). Sedate with low-dose ketamine (0.5 - 2 mg/kg) after an
antisialogogue (eg, glycopyrrolate).
If intubation is required, do an awake intubation so that positive pressure ventilation
can be avoided until surgeon performs gastrostomy. Avoid N2O.
Surgeon should use local anesthetic to facilitate quiet field.
Once the repair commences, you can attempt to occlude the fistula by positioning the
tip of the ETT such that the bevel covers the fistula. You can confirm correct
placement when bubbles cease coming out of the end of the submerged g-tube (see diagram
below).
DIAPHRAGMATIC HERNIA (DH): DH is relatively common (1 in 5000 births), usually a left
posterolateral defect (foramen of Bochdalek). There is a varying degrees of pulmonary
dysplasia. Mortality is as high as 50%. Intestinal malrotation (50%) and cardiovascular
defects (25%) are commonly associated with DH. A scaphoid abdomen and respiratory
distress should trigger suspicion in the neonate.
Anesthetic Management of DH:
Decompress herniated stomach preoperatively with gastric tube as soon as diagnosis is
suspected.
Avoid positive pressure ventilation by mask (inflate stomach -> further respiratory
compromise).
Intubate awake with gentle (25-30 cm H2O) positive pressure (vigorous ventilation may
result in PTX on either side).
Anesthetize with narcotic, low-dose potent inhalational agent, profound muscle
relaxation. Avoid N2O, which can distend bowel, or exaggerate a pneumothorax.
Assist surgeon in determining feasibility of primary abdominal closure. Since
abdominal cavity is small, it is sometimes necessary for the surgeon to create a
ventral hernia. This will be necessary if ventilating pressures of >30 cm are required
after abdominal closure.
Arterial line should be preductal (right radial or either temporal) if practical.
Postoperative management is chiefly centered about control of pulmonary artery
pressures, since with the underdeveloped lung (often on both sides) the cardiac output
is forced through markedly diminished vascular bed. Besides standard care to reduce
pulmonary vascular resistance (maintenance of moderate hyperventilation, avoidance of
acidosis) three therapeutic interventions may be considered:
Pulmonary vasodilation with tolazoline, 1mg kg followed by 5 mg/kg/hr (dopamine
may be needed to counteract systemic effects)
Ligation of a patent ductus arteriosus
More recently, ECMO (extra-corporeal membrane oxygenation) has proved to be the
most efficacious treatment for DH infants with refractory hypoxemia
DH is not a surgical emergency. Surgery should be undertaken only after the patient
has been satisfactorily stabilized. (Ref: Langer J, et al: Timing for surgery for
congenital diaphragmatic hernia: Is emergency operation necessary?. J Pediatr Surg
23;731-734:1988)
GASTROSCHISIS AND OMPHALOCELE: In these two defects, anesthestic management is
essentially identical. The most critical anesthetic problems are hypovolemia and
hypothermia.
Gastroschisis
Omphalocele
Intrauterine occlusion of omphalomsenteric
artery leads to full thickness abdominal
wall defect
Failure of gut to return to abdominal
cavity. A mucous sac overlies viscera.
Herniation into base of umbilical cord.
Never covered with membrane, so antenatal
peritonitis due to exposure to amniotic
fluid. Greater fluid and heat loss
postpartum
Usually covered with membrane which protects
bowel both before and after birth. Ruptured
omphalocele similar to gastroschisis from
anesthetic perspective.
Low incidence of associated defects. 10%
have malrotation or small bowel atresia.
Excellent prognosis.
High incidence of prematurity and congenital
defects, esp cardiac and bladder defects,
Beckwith syndrome (macroglossia, severe
hypoglycemia). Mortality of 30%.
Anesthetic Management of Gastroschisis/Omphalocele:
Decompress stomach with large bore N/G prior to intubation. Awake intubation usually
indicated.
Arterial line monitoring mandatory to monitor volume, acid-base status, and post-op
ventilation. A single very good IV acceptable, but two are preferable. Lines should
be placed in the upper extremities if possible, since the abdominal pressure may be
great enough postop to cause edema and stasis in the lower extremities.
Adequate hydration is the most critical factor. Immense amounts of protein-laden fluid
is lost to third space due to the peritonitis and the large amount of exposed and
surgically manipulated bowel. Large defects may require more that 100 ml/kg during the
operation and up to 300 ml/kg during the first 24 hours. Fluid should be a balanced
salt solution (eg, R/L) and Plasmanate. Avoid excessive glucose loads with the immense
amounts of fluids given.
Keep the infant warm. A Bair hugger is essential. IV fluids should be warmed.
Profound abdominal relaxation needed when the surgeon attempts to relocate the gut into
the constricted abdominal cavity. Administer a drip of either mivacurium (about 15
ug/kg/min) or cis-atracurium (about 3 ug/kg/min) throughout the main portion of the
case.
You will often need to help the surgeon decide whether or not he must construct a
temporary silo in which to house the gut until the abdominal cavity enlarges. If the
ventilating pressure rises to >30 cm H2O as he reduces the gut into the abdomen, then a
silo will be required.
Patients with all but the smallest of defects should be ventilated postop.
NECROTIZING ENTEROCOLITIS:
NEC is a disease of small (<1500 gm), premature (<32 weeks)
infants. Etiology is multifactorial. Infants exhibit lethargy, apnea, hypovolemia,
acidosis, and thrombocytopenia. Submucosal gas in the bowel wall is a classic
radiological finding. Infants that are treated surgically are gravely ill, with bowel
perforation, peritonitis, acidosis and severe hypovolemic shock. Most have some degree of
hyaline membrane disease.
Anesthetic Management of NEC:
Arterial line monitoring essential. Preductal (right radial or temporal) positioniing
of your arterial catheter is desirable, since many of these infants will have an open
PDA. Since the PA pressures are often high in these patients, blood will shunt right > left through the ductus, resulting in lower SAT's in the left arm and legs. If in
shock, axillary artery cannulation may occasionally be necessary, but be sure to remove
line as soon as feasible postop to avoid possible vascular complications to hand and
arm.
Avoid N2O, as it may increase bubble size in submucosa, possibly resulting in air
embolus.
Anesthesia usually consists of ketamine and/or narcotics, plus a mixture of air and
oxygen.
All these infants are returned to the NICU on full ventilatory support.
PYLORIC STENOSIS: Common (1:500) disorder, usually seen in male infants 4-6 weeks old.
Persistent projectile vomiting leads to hypochloremic, hypokalemic metabolic alkalosis.
Significant dehydration always present. In extreme cases, the dehydration will be so
severe that the alkalosis will be replaced by a metabolic acidosis.
Pyloric stenosis is never a surgical emergency. Surgery must be preceded by correction of
any significant fluid and electrolyte deficits. The criteria we use for acceptable
electrolytes preoperatively are: K >3.3, Cl > 90, bicarb <30. Skin turgor should be
good, and there should be evidence of return of urine output (wet diaper).
All these infants are at extreme risk for aspiration with induction. A history of a
barium swallow should always be elicited preoperatively, since the barium invariably is
still in the stomach at the time of induction and therefore adds to the risk of
aspiration.
Anesthetic Management of Pyloromyotomy:
Turn infant on side and empty stomach (awake) with large-bore catheter. Stomach
volumes in these patients are exceedingly large, averaging >5 ml/kg, and are not
influenced by either pre-operative NG suctioning nor by whether or not a barium swalow
was performed (ref 1).
Rapid sequence induction with preoxygentation and firm cricoid pressure is usually
employed in vigorous infants. In less vigorous infants, particularly in infants who
have had a barium study prior to coming to the OR, awake intubation should be
considered.
Potent inhaled agents are preferred over fixed agents (eg, narcotics), since return to
spontaneous ventilation immediately postop is desired.
Infants must be fully awake and vigorous, with normal ET CO2, prior to extubation,
since preexisting alkalosis may inhibit respiratory drive.
Muscle relaxation is essential as the surgeon enters abdomen to prevent a loop of bowel
or omentum from obscuring the exposure through the very small incision. Either a
supplemental dose of succinylcholine or the judicious use of mivacurium is appropriate.
The entire surgery only takes about 20 minutes, so in general use short-acting
relaxants and inhaled agents with low solubility to facilitate a rapid, safe emergence.
CONGENITAL LOBAR EMPHYSEMA: Usually a single lobe of the lung is affected, most commonly
the left upper. 10% of patients have coexisting congenital heart disease. The clinical
picture is one of progressive respiratory distress due to hyperinflation of the
emphysematous lobe with atelectasis of the contralateral lung. As the mediastinum is
shifted and the intrathoracic pressure rises, cardiovascular compromise becomes
significant.
Anesthetic Management of Congenital Lobar Emphysema:
Minimize positive pressure ventilation until thoracotomy decompresses the lungs. N2O
is absolutely contraindicated. Since surgical decompression may be rapidly indicated
following induction, the surgeon must be present during induction.
Ketamine, narcotics or inhaled anesthetics (no N2O) may be used, depending on the
degree of cardiovascular and pulmonary compromise. Narcotics have the disadvantage of
necessitating positive pressure ventilation before the incision, but the potent inhaled
agents may depress the cardiovascular system or may lead to more pulmonary shunting and
hypoxia.
Consider endobronchial intubation, especially in cases of left-sided lesions.
Arterial line monitoring indicated.
References:
1. Cook-Sather SD, et al:
Anaesth 1997;43:278-283.
5/99
TWolfe
Gastric fluid volume in infants for pyloromyotomy.
Can J
CARDIAC TRANSPLANTATION
THE TRANSPLANTED HEART: During cardiac transplantation, the donor atria are anastomosed
to the recipient's residual atria. This results in two autonomous atrial depolarizations
(p waves) on ECG. The intrinsic rate of the denervated rate is frequently quite low,
requiring chronotropic support to maintain more than 90 bpm in adults, or 120 bpm in
children. Dysrhythmias are not uncommon in the denervated heart. Dysrhythmias are
especially likely during the first six months following transplant, or during episodes of
rejection.
Intrinsic control mechanisms are maintained in the transplanted heart, including a normal
Frank-Starling response to volume loading, normal impulse formation and conduction, and
intact alpha and beta receptors without evidence of denervation hypersensitivity. The
transplanted heart responds atypically to stress as a result of the lack of autonomic
neural control of the SA node. Specifically, stress may not be manifest by an increase in
heart rate. Direct-acting drugs will affect the donor heart, but drugs that depend on
intact autonomic innervation will be ineffectual. Increases in cardiac output are
dependent on increases in venous return (preload) until circulating catecholamine levels
rise sufficiently to increase heart rate and contractility. Therefore, cardiac transplant
patients have a delayed response to stress and do not tolerate volume depletion.
INTRAOPERATIVE MANAGEMENT
Sterile technique
Equipment: use clean, single use items such as blood pressure cuffs and pulse oximeter
probes.
Lines: wear gloves, start lines after careful prep, change lines that are more than 12
hours old. Central lines already in place are maintained until the postoperative
period.
Central Line Placement
Central lines are not indicated for the majority of patients pre bypass.
If a central line is indicated and the size and anatomy of the patient permit, CVP
and/or pulmonary artery pressure monitoring can be established via the left IJ. The
right IJ is usually avoided, since the cardiologists may use that vessel for
posttransplant biopsies. Central monitoring can also be established femorally, if
necessary. Equipment for central line placement is located in two drawers in the
anesthesia workroom labeled "femoral line" and "cardiac transplant". The only other
equipment you would need is a sheath (for children < 20 kg), which are located in
overhead bins in the workroom. Because manipulation of the pulmonary artery catheter
is frequently necessary, a sterile sleeve must be placed prior to catheter insertion.
Following the transplant, the surgeon may place an oximetric RA catheter, a PA line,
and/or an LA line.
Anesthetic Technique: No single technique is best for all patients, but most transplant
patients will benefit from a narcotic-based anesthetic to minimize cardiac depression.
When anesthetizing neonates with hypoplasic left heart syndrome, the major hemodynamic
problem is maintaining the balance between the pulmonary and the systemic circulations,
since both are supplied by the common ventricle. Physiologic or pharmacologic
manipulations that decrease pulmonary vascular resistance may cause an increase in
pulmonary blood flow at the expense of systemic and coronary perfusion. In hypoplastic
left heart babies, keep the PaO2 at or near preoperative levels unless otherwise
indicated, since a high PaO2 may significantly reduce pulmonary vascular resistance,
thereby reducing systemic blood flow. A PaO2 of 40 mm Hg corresponds to an arterial SAT
of about 80%. This value is near the plateau of the fetal oxyhemoglobin dissociation
curve and is felt to provide adequate oxygen transport to the tissues (ref 1).
Non-anesthetic drugs given prior to bypass may include azothioprine (Imuran), cefuroxime
and ranitidine. These drugs and doses should accompany the patient to the OR from the
ward or emergency room. If the patient has had prior open-chest surgery, the surgeon may
want you to give 5 ml/kg of aprotinin, with a second dose by the pump technicians at the
onset of bypass.
Coming Off Bypass
Solumedrol 15 mg/kg is administered through the pump prior to removal of the cross
clamp.
Isoproterenol, dopamine and nitroglycerin are routinely infused when weaning these
patients from bypass.
Isoproterenol is used to maintain heart rate near the upper limits of normal,
based on age. The usual dose ranges from 0.02-2 ug/kg/min.
Dopamine is used for inotropic support and to improve renal perfusion, usually in
a dose of 2-5 ug/kg/min.
Nitroglycerin is indicated to reduce pulmonary artery pressure and as a coronary
vasodilator, usually in a dose from 0.5-2 ug/kg/min.
Elevated Pulmonary Artery Pressures are the most lethal perioperative problem. In
addition to hyperventilation and aggressive treatment of acidosis (pH should be >7.45, and
often in the range of 7.5), pharmacologic options include:
milrinone 50 ug/kg followed by 0.5 ug/kg/min (ref 3)
PGE1 (alprostadil) 0.030-0.150 ug/kg/min
nitroprusside 0.25-5 ug/kg/min
tolazoline 1-2 mg/kg/hr, or 16-33 ug/kg/min
nitric oxide
Direct infusion of vasodilators into the pulmonary artery may be efficacious in malignant
pulmonary hypertension.
INTENSIVE CARE MANAGEMENT
Intensive care of the transplanted patient is a continuation of the principles begun in
the operating room. These include:
vigorous hyperventilation
aggressive treatment of acidosis
minimization of alpha effects of inotropes
analgesia with fentanyl, usual dose range 5-15 ug/kg/hr
hyperoxygenation until patient hemodynamically stable
Extubation: OKT-3 is currently first administered 24 hours following the transplant.
Therefore, consideration should be given to delaying extubation until 24 hours postoperatively, after the first dose of OKT3. OKT3 is a murine-derived antibody that, in
combination with the CD3 antigen, selectively blocks helper T3 cells. Its administration
activates the complement system. The first dose often results in significant hypotension
and pulmonary edema.
DRUGS AND THE DENERVATED HEART
Inotropic Support: epinephrine, dopamine, isoproterenol and milrinone are effective
Dysrhythmia Therapy
atropine has no effect
digitalis not effective for control of acute atrial fibrillation (primarily vagally
mediated), but may be beneficial in chronic AF
lidocaine useful, but reduce dose with impaired hepatic function
phenytoin useful, but increases rate of cyclosporin metabolism
quinidine, procainamide useful
Drugs with No Effect
pancuronium
cholinergic antagonists, eg atropine
anticholinesterases, eg neostigmine
REFERENCES:
1. Clark N, Martin R: Anesthetic considerations for patients undergoing cardiac
transplantation. J Cardiothorac Anesth 2:519-542,1988.
2. Martin R, Parisi F, et al: Anesthetic management of neonatal cardiac transplantation.
J Cardiothorac Anesth 3:465-469,1989.
3. Chang AC, et al: Milrinone: systemic and pulmonary hemodynamic effects in neonates
after cardiac surgery. Crit Care Med 1995;23:1907-1914.
4. Montenegro LM, et al: New directions in perioperative management for pediatric solid
organ transplantation. J Cardiothor Vasc Anesth 1998;12:457-472.
9/98 TWolfe
LIVER TRANSPLANT
PATIENT EVALUATION: The typical patient is a 1 yr old and weighs 10 kg. Biliary atresia
is the usual indication. While many other physiologic derangements will be noted, look
specifically for coagulopathy as this may complicate line placement.
SURGICAL PROCEDURE: There are 3 phases: (1) The "Mercedes-Benz" incision is made and
the diseased liver is dissected out.
(2) The anhepatic phase, when the vascular supply
to the liver (IVC, portal vein and hepatic artery) is clamped and the liver is removed.
Vascular anastomoses are made to the new liver. Sometimes the hepatic artery will be
anastomosed to the aorta directly which requires aortic occlusion. The graft is
reperfused by removal of the vascular clamps. (3) The biliary tract is reconstructed and
the abdomen closed.
Occasionally in children, a lobectomy is performed ex vivo on an adult size graft and the
lobe is then implanted into a child. Closure is usually tight, which may impair postop
ventilation and visceral perfusion.
INDUCTION/INTUBATION/MAINTENANCE: Patients can be induced with thiopental since they are
usually hemodynamically stable. Since intragastric pressure may be elevated due to
ascites, a rapid sequence induction with succinylcholine may be desirable. Maintenance is
with an inhaled anesthetic. If moderate levels of an inhaled agent are not tolerated,
blood administration to correct hypovolemia is indicated. Nitrous oxide is avoided to
prevent bowel distension and to minimize risk of air emboli.
VENOUS LINES: Ideally, lines should be placed in vessels draining above the diaphragm,
since the vena cava will be clamped. Two large peripheral venous lines are needed, as
well as a central line for measurement of CVP and for drug infusions. Level 1 blood
warmers are connected to the largest lines for blood infusions. Blood is infused using
self-inflating pressure boxes (Alton Dean) and Y-type blood administration sets.
Large peripheral lines can be obtained by first placing a 20 gauge catheter, then dilating
up to either 7 or 8.5 French.
By applying chux and plastic drapes over the arms after venous and arterial line
placement, you can prevent the tape from becoming soaked loose by blood from the field.
LABORATORY PROTOCOLS:
Blood gases hourly, including Hct, Ca++, K+, Na+, glucose
Coags at beginning of case (if not done preop), just after unclamping new liver, and close
to end of case
Platelets in lavender tube
PT, PTT, fibrinogen, D-dimer, INR in blue tube
SPECIAL ANESTHETIC CONSDIERATIONS:
Bleeding: Losses can exceed 2 blood volumes, though current surgical techniques have
greatly reduced average blood loss. PRBC and FFP infused 1:1 (mixed with Y setup).
Calcium gluconate 100 mg/50 ml of either FFP or platelets should be given to counteract
chelation by citrate. Packed cells have little citrate, so calcium is less necessary to
give when they are infused. Monitor Ca++ closely.
Cardiovascular: Most problems result from hypovolemia and hypocalcemia. A large loss of
preload can be anticipated with caval clamping, and is treated with volume loading.
Potassium-rich, acidotic perfusate and other vasoactive substances from the GI tract are
released when the graft is reperfused resulting in combined myocardial depression and
peripheral vasodilation. Treat with hyperventilation, infusion of calcium and
bicarbonate, volume, and sometimes a small dose of epinephrine (ref 4). Keep diagnosis of
air embolism in mind – watch for acute decreases in end-tidal CO2.
Coagulation: Dilutional thrombocytopenia, fibrinolysis, and heparin release from the
graft are major contributors to coagulopathy. Treatment is based on serial lab tests (PT,
aPPT, platelets and fibrinogen). If the PT is markedly prolonged, consider FFP
administration. If the aPPT is much more prolonged than the PT, then get an ACT to check
for heparin effects. If ACT is prolonged, consider protamine 1-2 mg/kg. If the
fibrinogen is <150, consider cryoprecipitate or, if fibrinolysis is suspected, Amicar.
Renal: Oliguria is common during caval occlusion. Dopamine is infused at 3-5 ug/kg/min
throughout and optimize preload. Mannitol, if required, should be run at 0.05 - 0.1
gm/kg/hr.
Fluids/Metabolic: Crystalloid (Plasmalyte) infused at 10+ ml/kg/hr. Consider using
colloid if COP < 15. One IV should be D5RL at maintenance (4 ml/kg/hr), the rest Normosol
R (w/o dextrose and w/o calcium). Hyperglycemia is the rule, but hypoglycemia has been
encountered. Check glucometer hourly.
Hypothermia: A steady decline in temperature occurs due to the wide exposure of
peritoneal surfaces, with a sudden drop occurring when heat is transferred to the new
liver. Fluid warmers and a Bair Hugger are necessary.
Pulmonary: Be alert for pulmonary edema, pneumothorax, and air embolism intraoperatively.
Pulmonary compliance may fall unacceptably during closure when the abdomen is too tight.
If inflating pressures exceed 40 cm H2O in the paralyzed patient, then consideration may
be given to splenectomy to facilitate closure, and sedation and paralysis continued
postoperatively.
Laboratory Protocols:
Blood gases hourly, including Hct, Ca++, K+, Na+, glucose
Coags at beginning of case if not done preop, just after unclamping new liver, and close
to end of case
Platelets: lavender tube
PT, PTT, fibrinogen, D-dimer, INR: blue top tube
Steroids: A bolus dose of steroids, 10 mg/kg of SoluMedrol (methyprednisolone), is given
prior to reperfusion. (Note -- dose may vary with different surgeons.)
Aprotinin: Before incision, give 7 ml/kg, followed by a drip throughout the case of 1
ml/kg/hour.
OKT-3: Infused per surgical request.
POSTOPERATIVE: Most patients are ventilated for a few hours to a few days post-op.
Weaning is complicated by fluid retention (due in part to cyclosporine) and tight
abdominal closure.
SELECTED REFERENCES
1. Borland LM, Roule M, Cook DR: Anesthesia for pediatric orthotopic liver
transplantation. Anesth Analg. 1985;64:117-24
2. Kang YG, et al: Intraoperative changes in blood coagulation and thromboelastographic
monitoring in liver transplantation. Anesth Analg 1985;64:888-96
3. Tompson AE. Aspects of pediatric intensive care after liver transplantation.
Transplant Proc1987;19(4, suppl 3):34-9
4. Carton EG, et al: Perioperative care of the liver transplant patient: Part 2.
Anesth Analg 1994;78:382-99.
TWolfe
Feb 2002
RENAL TRANSPLANTATION
PATIENT EVALUATION: Patients with chronic renal failure exhibit multiorgan failure, so a
thorough preoperative evaluation is essential to avoid nasty intraoperative surprises.
These patients are usually on steroids, may be insulin dependent, are often on multiple
drugs to control labile hypertension, are usually anemic and hyperparathyroid, and may be
either hypo- or hypervolemic. They have delayed gastric emptying, and they exhibit
exaggerated and/or prolonged responses to many anesthetic drugs.
Preop Checklist:
Adequacy of dialysis
1. Volume status
2. Acid-base status
3. Electrolytes, especially K
Hbg concentration: chronic anemia anticipated
Cardiovascular status: labile hypertension often present
Metabolic status: insulin-dependent diabetes often present
Coagulation studies and platelet count
INDUCTION / INTUBATION / MAINTENANCE:
Ideally, patients should be NPO 6-8 hours, since
gastric emptying is delayed. Consider antacids (15-30 ml of Bicitra PO), H-2 blockers
(cimetidine 7.5 mg/kg IM), or metoclopromide (0.1 mg/kg IV).
Titrate induction agents
slowly to avoid hypotension. Beware of potential aspiration risk on induction. Inhaled
agents, narcotics, ketamine, and non-depolarizers (esp cis-atracurium) are all appropriate
for maintenance. Theoretically, regional anesthesia is acceptable, but should be avoided
in renal patients who have a neuropathy or decreased platelet function.
MONITORING / VENOUS LINES: In addition to the standard monitors, an arterial line, a CVP.
and a Foley catheter are required. Usually, the general surgeons will place a central
line at the onset of the case that we can use for CVP monitoring. Exercise strict aseptic
technique for lines in immunosuppressed patients. Lines should not be placed in the lower
limbs, distal to where a common iliac vessel may be clamped.
FLUIDS: Induction fluids can be any balanced salt solution without K. D5NS running at 4
ml/kg/hr is appropriate. After the new kidney is implanted, the transplant surgeon will
want you to maintain a very high CVP (10+ cm of H2O) to insure good perfusion of the newly
implanted kidney. In general, this is done with NS and an infusion of 5% albumin.
Warming of infused fluids is essential, since immense amounts of fluid may be needed to
achieve the desired filling pressures. Also, the implanted kidney may be quite cold,
which will impose another thermal stress when it is perfused.
SPECIAL DRUGS per Dr Pescovitz (may vary for other surgeons). Give early in the case,
prior to onset of surgery.
1. Methypredisolone (5-10 mg/kg) as soon as IV is in. Helps prevent ischemic reperfusion
injury.
2. Cefuroxime 25 mg/kg
3. Daclizumab (Zenopax) 1-2 mg/kg. Monoclonal antibody to CD-25 (interleukin 2).
Prevents WBC’s from rejecting kidney. Give over approximately 15 minutes (rate not
critical). Comes in 25 mg amps, so give 1 mg/ kg up to 2 mg/kg if that does not involve
opening another (expensive) amp. Example: give 2 amps to patient who weighs 30 kg, or
give 1 amp to patient who weighs 20 kg.
4. Mycophenolate mofetil (Cellcept) 600 mg/m2 (dose based on body surface area).
Cellcept works by blocking DNA synthesis. Must give over 2 hours due to risk of
phlebitis. Give in central vein.
ENHANCEMENT OF URINE OUTPUT: There are several things you should be prepared for, besides
volume loading, to enhance the perfusion and urine output of the newly implanted kidney:
volume loading as above, to a CVP of 10 to 12 cm H2O
osmotic diuresis with mannitol, 0.5 gm/kg
diuresis with furosemide, 1 mg/kg
“renal-dose” dopamine, up to 5 ug/kg/min
MUSCLE RELAXATION / REVERSAL: With the titrated use of the intermediate-acting muscle
relaxants, muscle relaxation can be safely reversed at the end of the procedure using any
of the standard anticholinesterase-anticholinergic combinations. Use of cis-atracurium,
which does not rely on renal or hepatic elimination, is logical.
ABDOMINAL CLOSURE / POSTOP VENTILATION: In small children, occasionally a kidney will be
implanted abdominally that is so large that the abdomen cannot be closed without tension.
Tight closure may compromise the blood supply to the new kidney and also retard venous
return from the lower extremities. In addition, if the closure is very tight, ventilating
pressures may be increased to the point where a period of post-operative ventilation may
be required. In general, if peak ventilating pressures exceed 30 cm of H2O, then serious
consideration should be given to the need for postop ventilation.
Also, when a large kidney is transplanted into a small infant (usually a living related
donor), the new kidney will require a large proportion of the total cardiac output. The
SVR, and therefore the BP, will fall when the clamps are removed and the large vascular
bed of the new kidney is perfused, so the volume status must be optimized.
TRANSFUSION: Most of these patients tolerate a chronic anemia well, so transfusion often
depends on whether or not you have increasing signs of inadequate tissue oxygenation, such
as a worsening base deficit. If transfusion is required, use packed RBC’s through a
leukocyte filter to reduce risk of graft-vs.-host disease. (The leukocyte-filtered blood
is currently favored over irradiated packed RBC’s.) Unfortunately, transfusion through
leukocyte filters is extremely slow.
updated 3/05
TWolfe
BONE MARROW TRANSPLANTATION
RECIPIENT: Bone marrow recipients undergo a preoperative regimen designed to achieve
functional bone marrow ablation. This is done over 7-10 days, using combinations of
chemotherapy and radiation. At that time, rescue with transplanted marrow infused IV is
then begun. Time to engraftment is generally 10-28 days. Hematological support in the
form of platelets and RBC’s (leukocyte poor, irradiated) is usually necessary to keep the
platelet count >20,000 and Hct > 25. The bone marrow infusion does not need to be done in
the OR, though these patients often present to the OR for other reasons. Some of the
specific chemotherapeutic problems associated with these patients include:
Cisplatin
Daunorubicin
Bleomycin
Radiation Rx
Renal insufficiency
Congestive cardiomyopathy (exercise tolerance, MUGA)
Pulmonary insufficiency (limit fluids, limit FiO2)
Pulmonary fibrosis
After transplant, these patients are very susceptible to graft-vs.-host disease (GVHD),
manifest by GI problems that may include candidiasis, esophageal or gastric ulcers,
intractable diarrhea or bleeding. Hepatic insufficiency from either GVDH or venocclusive
disease is common. Prominent skin lesions from GVHD are common. Obstructive pulmonary
defects may result from GVHD-induced bronchiolitis, while restrictive defects are more
likely due to specific chemo or radiation therapy.
GVHD (summary):
Cutaneous
Impaired thermoregulation
Scleroderma-like syndrome
Ulceration and infection
Eye
Cataracts
Gastrointestinal
Diarrhea with fluid/electrolyte/blood loss
Esophageal infection and ulceration
Oral ulceration and propensity for invasive candidiasis
Hepatic
Acute and chronic hepatitis
Marrow
Pancytopenia and immunodeficiency
Pulmonary
Bronchiolitis obliterans
Interstitial pneumonitis
Pulmonary fibrosis
Renal
Insufficiency with electrolyte abnormalities
Renal tubular acidosis
ANESTHETIC MANAGEMENT:
Use extreme measures to assure asepsis
Avoid unnecessary nasal, rectal and urethral probes, and avoid esophageal suctioning or
stethoscopes in patients with esophagitis.
Anesthetic technique otherwise based on condition of patient and anticipated surgery
DONOR:
Children are frequently brought to the OR to be bone marrow donors for a relative.
Anesthetic Management:
Patient will be prone
Up to 1.5 liter of marrow (20 ml/kg) may be harvested from posterior iliac spines and
iliac crests. These marrow spaces are in dynamic equilibrium with the extracellular
fluid compartment. Therefore, sizeable volumes of fluid replacement and/or transfusion
(usually with autologous blood) may be required.
Some authors (ref 3) suggest that, following hemodilution at the beginning of the case,
blood can be withdrawn and then re-infused after the marrow has been harvested.
Nitrous oxide inactivates vitamin B12 which can then interfere with DNA synthesis.
However, recent work indicates that nitrous oxide does not interfere with bone graft
viability, and therefore may be used as part of your anesthetic technique (ref 4).
References:
1. Stein RA, et al: Anaesthetic implications for bone marrow transplant recipients.
Can J Anaesth 1990;37:571-8
2. Perez De Sa V, et al: Hemodilution during bone marrow harvesting in children.
Anesth Analg 1991;72:645-50
3. Perez De Sa V, et al: Bone marrow harvesting in children managed without allogenic
blood. Paediatric Anaesthesia 1994;4:375-381.
4. Lederhaas G, et al: Is nitrous oxide safe for bone marrow harvest? Anesth Analg
1995;80:770-772.
Updated 4/95
TWolfe
JUVENILE (TYPE I) DIABETICS
Anesthetic Management for the Diabetic for Elective Surgery
Goal: maximize metabolic control prior to surgery. Normal insulin requirements are
0.7-1.2 U/kg/day, and may increase to around 2.4 U/kg/day in stressed patients with
ketoacidosis.
For most short surgeries, plan procedure for first thing in the morning. Patients can
follow their usual regimen until the day of surgery.
When patient arrives in Day Surgery, check glucose level to rule out hypoglycemia or
gross hyperglycemia. This can be done by the patient with their own glucose monitor or
with our glucometer.
Check recent glycosylated hemoglobin (Hb-A1c). Normal 4-6%. Increased levels indicate
poor control for previous 1-3 months (ref 2).
If glucose in reasonable range (80-250) begin IV infusion with D5RL at a maintenance
rate. Administer 2/3 of the usual subcutaneous NPH, Lente or Humulin Ultralente for
patients on 2 daily injections. Follow glucose hourly during surgery.
Give supplemental Humalog insulin every 6 hours if glucose > 250. If glucose < 250, do
not give insulin. Humalog has an almost immediate onset of action, and lasts
approximately 2 hours.
Pediatric Endocrinology (4-3889) is always available to help you plan the optimal
preoperative management of any individual patient.
Supplemental Short-Acting Insulin for Patients Who Cannot Eat (including patients for
prolonged surgeries)
In general, if the intraoperative blood glucose is >250, an insulin infusion (as
opposed to intermittent intravenous insulin injections) may be required. The starting
dose in non-ketotic or minimally ketotic children should be about 0.02 U/kg/hr of
regular or Humalog insulin with a 5% dextrose drip running at maintenance
(approximately 1 unit per 5 to 6 grams of glucose). Hourly blood sugar measurements
are essential. If necessary, the insulin infusion can be incrementally increased to as
high as 0.1 U/kg/hr. Factors that would tend to favor lower rates of insulin infusion
include newly diagnosed patients, younger patients, and those with no or minimal
ketones. A faster rate of infusion may be needed in patients who are stressed, on
steroids, or receiving hyperalimentation fluids.
An alternative method of insulin administration in children whose glucose exceeds 250
is to administer subcutaneous insulin. In non-ketotic children, administer 0.15 U/kg
of Humalog insulin SQ; if ketotic, administer 0.2 U/kg SQ.
In any patient requiring an insulin infusion or with any degree of metabolic acidosis,
strongly consider obtaining a consult with Pediatric Endocrinology, phone 4-3889, both
to optimize intraoperative management and to assure a smooth transition to the
postoperative care.
References:
1. Hirsch I, et al: Perioperative management of surgical patients with diabetes
mellitus. Anesthesiology 1991;74:346-59
2. Zulfiqar A, et al: Advances in diabetic management: implications for anesthesia.
Review article in Anesth Analg 2005;100:666-9
TWolfe
6/2005
MUSCULAR DYSTROPHIES
Duchenne’s Muscular Dystrophy: The muscular dystrophy of most interest to pediatric
anesthesiologist is Duchenne’s muscular dystrophy (DMD). DMD is the severest form of the
pediatric muscular dystrophies. It is an X-linked recessive trait, so virtually all of
the children with this disorder are male.
Diagnosis is usually made in early childhood (preschool age group), and presents with
hypotonia and muscle weakness. Walking is often delayed. Pseudohypertrophy of the calf
muscles is sometimes seen. Proximal muscle weakness is characteristic, with sparing of the
cranial nerves.
Clinical cardiomyopathy is usually apparent by the second decade, and may progress to a
fatal dilated cardiomyopathy.
Diagnosis relies on the history and physical examination, creatinine kinase levels, and
the results of muscle biopsies and electromyography. More recently, DNA analysis can
detect about two-thirds of patients.
These patients come to anesthetic attention for a variety of reasons, particularly
scoliosis and other orthopedic surgeries, central line placements, bronchoscopies, and
tracheostomies in the end stages of the disease.
Specific anesthetic considerations include:
1. While no conclusive evidence for a link between DMD and malignant hyperthermia exists,
enough anecdotal evidence suggests that the use of a non-triggering anesthetic is
indicated if there is no clear-cut advantage of using an inhaled technique. However, most
anesthesiologists would still use an inhaled anesthetic if truly indicated, for instance,
for intubation of a DMD patient with a difficult airway where spontaneous breathing with
an inhaled agent might enhance airway safety.
2. Especially in end-stage disease, significant cardiomyopathy may be present, and a
preoperative ECHO may be indicated.
3. Most DMD patients develop severe restrictive lung disease and eventually succumb to
respiratory failure, or become totally ventilator-dependent. Some degree of postoperative
respiratory support is required for all but minor procedures in relatively early-stage
disease.
4. Succinylcholine3 should be avoided, not only because of the risk of MH but also from
the risk of hyperkalemia.
Mitochondrial myopathies:
related conditions.
The mitochondrial myopathies encompass a wide spectrum of
From the anesthetic perspective, things to know:
1. Fluids: avoid lactate, administer glucose. Ideal fluid D5NS or D51/2NS
2. Avoid propofol, at least in large doses
3. These patients are NOT at risk for malignant hyperthermia (source: MHAUS, 2005).
However, very rarely these patients may have other associated conditions that may be
associated with MH.
4. There is some suggestion that patients with mitochondrial myopathies may be sensitive
to the effects of inhaled anesthetics, so that careful titration of your anesthetic is
required.
References:
1. Systemic Disorders Commonly Seen in Pediatric Anesthesia, Chapter by T Yemen, J
Jaeger, in Pediatric Anesthesia Handbook, McGraw-Hill 2002, pp 288-291
2. Ciccotelli K K, et al: An adult with inherited metochondrail encephalomypathy: report
of a case. Aneshesiology 1997;87:1240-1242
SICKLE
CELL DISEASE
INTRODUCTION: Sickle cell disease (SCD) is a genetic disease involving a mutation in the
B-globin chain of the hemoglobin molecule. In the African American population, the
incidence of sickle cell disease is about 0.25%, while the incidence of sickle cell trait
is about 10%. The mutation in the B-globin subunit causes the hemoglobin to polymerize,
deforming the red cell into the classic crescent shape. This polymerization occurs when
the hemoglobin is in the deoxygenated form, and is initially reversible with oxygenation
of the RBC (ref 1). However, repeated polymerization leads to RBC membrane damage with
loss of intracellular water and potassium. The reticuloendothelial system removes the
damaged RBC’s from circulation, resulting in a chronic, hemolytic anemia.
Sickling occurs at an oxygen saturation below 80% (PaO2 of 50 mmHg). Consequently,
sickled cells are normally present in mixed venous blood. Sickled RBC’s increase plasma
viscosity and increase the adhesiveness of RBC’s, therefore decreasing tissue perfusion
and oxygenation, which in turn can lead to increased sickling. Hyperthermia and acidosis
promote sickling. Dehydration, by raising plasma osmolarity and thereby dehydrating
RBC’s, also increases the risk of sickling.
Clinically, pulse oximetry is an accurate and very useful measure of Hb oxygen saturation
in patients with SCD. The oxyhemoglobin dissociation curve in these patients is shifted
to the right, meaning that Hb SS has reduced affinity for oxygen. This implies that at
any given SaO2, the expected PaO2 will be somewhat higher than normal.
An anesthetic
technique that insures SaO2’s in the upper 90’s should be selected, both intraoperatively
and in the recovery room. In extremes of desaturation and/or anemia, the pulse oximeter
tends to overestimate the degree of desaturation. Arterial blood gases should be measured
if there is any doubt of tissue oxygenation.
Surgeries related to the pathophysiology of SCD that are commonly performed in afflicted
children include splenectomy, gall bladder surgery, and surgery for leg ulcers. Other
surgical procedures include treatment of priapism, cautery procedures for epistaxis, and
orthodontic treatment for excessive growth of maxillary teeth.
For children of African descent presenting for elective surgery, parents should be asked
if the child has sickle cell disease. A CBC and reticulocyte count should be sent
preoperatively on children suspected of having SCD. If the child was born in Indiana
after 1985, then by law hemoglobin electrophoresis was performed at birth. These results
are available from the Newborn Screening Lab in Riley Hospital, phone 274-2231. If
suspicion of SCD exists and no Hb electrophoresis test has been done, a sickle cell screen
can quickly detect Hb S levels of >10%. At the same time that the screen is sent, blood
should be sent for Hb electrophoresis, since a subsequent transfusion will invalidate this
more definitive test for several months.
If a transfusion is anticipated either preoperatively or intraoperatively, the blood bank
should be notified that the patient has SCD, and an extended antigen match should be
performed to lessen the risk of allo-immunization. Patients with SCD are of African
descent, and more likely to have a transfusion reaction to some of the minor antigens when
transfused with blood from incompatible (usually Caucasian) donors (ref 2).
Children under the age of 5, or who have undergone splenectomy, will be on prophylactic
antibiotics, primarily for streptococcal infection. In these patients, antibiotic
coverage should be continued perioperatively, or supplanted with an alternative antibiotic
(a penicillen, cephalosporin, or macrolide). Routine SBE prophylaxis is not required for
dental surgery. Extremely ill patients will likely be receiving vancomycin until culture
results and sensitivities are known. The “Red Man syndrome” (vasodilation and extreme
hypotension) is greatly exaggerated when a vancomycin infusion is combined with the
effects of general anesthesia. Vancomycin, if given at all during administration of
general anesthesia, should be given very slowly (2 hours per dose) with careful
monitoring.
The most common serious postoperative complication is the acute chest syndrome,
consisting of chest pain, cough, hemoptysis, fever and pulmonary infiltrate. This often
signals a pneumonia, pulmonary infarct, or both. Therefore, careful attention to
pulmonary toilet and prevention of intraoperative and postoperative atelectasis is
essential. As in other types of surgery, adequate pain relief postoperatively may help
maintain pulmonary mechanics, especially following intraabdominal or thoracic surgery.
Patients with sickle cell trait
are not at increased risk for surgery and anesthesia.
PERIOPERATIVE CONSIDERATIONS in CHILDREN with SCD:
History: A past transfusion may identify patients at risk for a delayed transfusion
reaction. History of previous acute chest syndrome should alert the anesthesiologist
to the likelihood of postoperative pulmonary complications.
Physical Exam: Pulmonary exam should focus on evidence of pneumonia; cardiac exam
should focus on hepatosplenomegaly and signs of congestive heart failure.
Labs: CBC with reticulocyte count preoperatively. Other tests as dictated by surgery.
Preoperative Hydration: The goal is to avoid dehydration, particularly loss of red
cell volume. For elective cases, continue clear fluids up to 2 hours preoperatively.
If that is not feasible, begin an IV preoperatively. Use of a hypotonic fluid (eg, 1/2
NS) has the theoretical benefit of promoting movement of fluids into the RBC’s.
Transfusions:
For all but minor procedures, current guidelines call for preoperative transfusion
to a Hb >10, without regard for the resultant Hb S levels. An exchange transfusion
should be considered for a child whose Hb is already > 10. For a child undergoing
cardiopulmonary bypass, more aggressive transfusion criteria are indicated.
Transfusion is usually deferred for minor procedures (eg, ear tubes, hernia repair).
(ref 6)
When blood is set up, an extended antigen match should be done as part of the T&C.
During blood administration, the anesthesiologist should be particularly alert for a
hemolytic transfusion reaction.
Antibiotics
Prophylactic antibiotics are indicated for children who are on preoperative
antibiotics, or children who have had a splenectomy. Routine SBE prophylaxis is not
recommended for routine dental surgery.
Beware the “Red Man” syndrome if vancomycin is infused during anesthesia.
Monitoring: Besides routine monitoring that includes pulse oximetry and temperature,
arterial line monitoring should be considered for major surgeries as a confirmation of
the accuracy of pulse oximetry, to monitor the acid/base status, and to provide ready
access for sampling for hemotocrit and for tests of coagulation.
Choice of Anesthetic Technique: No specific anesthetic regimen has been shown to offer
specific advantages. In particular, there is no specific advantage of regional vs
general anesthesia (ref 4).
Temperature Regulation: Normothermia is the goal. Hyperthermia is associated with
increased sickling, and hypothermia may lead to peripheral vasoconstriction and
diminished peripheral blood flow.
Pulmonary Function: Care should be exercised to maintain good pulmonary toilet and to
maintain lung volumes. The most common postoperative complication is the acute chest
syndrome (see above).
Tourniquets: Tourniquets may be used for extremity surgeries. The limb should be
thoroughly exanguinated prior to tourniquet inflation, and tourniquet time should be
minimized.
Postoperative analgesia: Patients with SCD may require relatively large doses of
narcotics if they have been on narcotics preoperatively for management of a painful
crisis. Adequate analgesia following abdominal or thoracic surgery will help maintain
postoperative pulmonary function.
REFERENCES:
1. Stasic AF: Anesthetic implications of sickle cell anemis. Progress in Anesthesiology
1994;8:3-28.
2. Vichinsky EP, et al: Alloimmunizatioin in sickle cell anemia and transfusion of
racially unmatched blood. NEJM 1990;322:1617-1621.
3. Rackoff WR, et al: Pulse oximetry and factors associated with hemoglobin oxygen
desaturation in children with sickle cell disease. Blood 1993;81:3422-3427.
4. Koshy M, et al: Surgery and anesthesia in sickle cell disease. Blood 1995;86:36763684.
5. Romanelli VA, et al: Intraperative and postoperative effects of vancomycin
administration in cardiac surgery patients: A prospective, double-blind, randomized
trial. Critical Care Med 1993;21:1124-1131.
6. Firth PG, Head A: Sickle cell disease and anesthesia. Anesthesiology 2004;101:76685. Excellent review of SCD.
11/04
TWolfe
ANESTHESIA for BURN PATIENTS
Burns are very common in our society. Two million people are treated for burn injuries
every year, and nearly 5000 children die from burn injuries.
INITIAL EVALUATION AND TREATMMENT: The initial evaluation of the acutely burned patient
should focus on 3 areas: respiratory, hemodynamic, and percent body surface area (BSA)
burn.
Respiratory:
Carbon monoxide and/or cyanide toxicity: CO and CN are both common components of
smoke. Any burn patient who was caught in a confined space should be suspected of
having inhaled both CO and CN. CO is toxic in two ways. First, it binds Hbg 240 times
as avidly as oxygen, therefore lowering oxygen carrying capacity. Pulse oximetry will
not distinguish carboxyHbg from oxygenated Hbg, since the light absorbtion
characteristics of CO-Hb are the same as oxyHbg. However, CO levels can be obtained
from a blood gas sample by checking the appropriate box on the blood gas requisition,
since most blood gas labs are equipped with co-oximeters, which can quantify CO-Hb.
Second, both CO and CN act by interfering with oxidative phosphorylation in the
electron transport chain at the cellular level. Although CN levels can be obtained by
sending a 10 ml gray-topped tube to the main lab clearly marked “Cyanide Level” ( not
thiosulphate level), levels will not be reported for 24-48 hours. Therefore, any
patient involved in a fire with neurologic signs such as headache, confusion, seizures
or coma should be suspected of carbon monoxide and/or CN toxicity and treated with the
highest practical FiO2, regardless of the patient’s PaO2 or pulse oximeter reading.
Smoke Inhalation Syndrome: Inhalation of smoke causes a chemical pneumonitis not
unlike aspiration pneumonia. Burn victims with a decreased oxygen saturation on room
air, wheezes, bronchospasm, and/or carbonaceous sputum have smoke inhalation syndrome
until proven otherwise. Initial treatment includes early intubation, early initiation
of PEEP, vigorous pulmonary toilet including bronchoscopy, and serial monitoring of
arterial blood gases. As with aspiration pneumonia, the early clinical and
radiographic picture may look fairly benign, only to worsen rapidly over the course of
a few hours.
Upper Airway Obstruction: Patients with burns about the head and neck are at extreme
risk for upper airway obstruction as the tissues swell following vigorous volume
resuscitation. In these patients, the airway should be secured very early in the
course of treatment (Am J Anesthesiol 1995;22:263-265). Nasal intubation may be
desirable if technically possible, since it is easier to secure and less uncomfortable
for the patient.
Hemodynamics:
The cornerstone of acute burn management is aggressive fluid resuscitation, initially
estimated by a guideline such as the Parkland burn formula:
ml crystalloid for first 24 hours = 4 ml/kg
X
% BSA burn
X
kg weight
Half of the above fluid is given in the first 8 hours. The Parkland formula is a
guideline, and must be modified for the individual patient and changing conditions. The
adequacy of fluid resuscitation is judged by watching the urine output, serial base
deficits, and serial CVPs. Plasmanate or other colloid can be used for a portion of the
initial fluid resuscitation. Concurrent smoke inhalation injuries increase fluid
requirements, sometimes dramatically.
% BSA Burn:
An estimate of the percent BSA burn is important because it helps estimate fluid
requirements, metabolic needs, and prognosis. The “Rule of Nines” used in adults must be
modified in children, particularly to take into account the relatively greater surface
area of the child’s head:
ANESTHETIC CONSIDERATIONS IN BURN PATIENTS
Choice of Anesthetic: The choice of agent for general anesthesia is not nearly so
important as the skill with which the chosen anesthetic is given. Burn patients have a
very high degree of variability in factors that effect pharmacokinetics, such as
protein binding and clearance rates. Likewise, they also may have been exposed to many
drugs and conditions that may affect pharmacodynamics. And finally, they may have
alterations in cardiac output and volume status that have implications for the choice
of agent. All of this means that the burn patient’s reaction to any given anesthetic
may be unpredictable, and careful titration of the chosen drug is essential.
Ketamine: Ketamine has some unique properties that make it useful in burn patients.
Ketamine is an excellent somatic analgesic even at subanesthetic doses, and under light
levels spontaneous ventilation is well preserved, so it is often used for such
procedures as dressing changes. It often allows the patient to be moved and dressings
changed without being encumbered by an endotracheal tube. Ketamine also is useful for
inducing the critically ill and perhaps hypovolemic burn patient, since it tends to
support the cardiovascular system.
Muscle Relaxants: Succinylcholine given in the days and weeks following a major burn
injury results in hyperkalemic cardiac arrest. While succinylcholine may be used
safely in the first few hours following a burn, it is contraindicated thereafter for a
period of at least months, or until all wounds are well healed. Non-depolarizers are
very useful in burn patients, but due to spread of extrajunctional end-plates, the dose
requirement of non-depolarizers is often increased dramatically. Burn patients
typically require more than double the normal dose of non-depolarizer, and sometimes up
to five times the normal dose
Blood Loss: During tangential excisions, the surgeon shaves off layers of tissue
“tangentially” until he reaches viable tissue. The surgeon identifies viable tissue
because it bleeds. Blood loss during tangential excisions is often massive, averaging
from about 1/2 to 2 blood volumes. When the blood loss has approached one blood
volume, it is prudent to ask the surgeon if further excision can be put off until
another day to avoid coagulopathy, hypothermia, and hemodynamic instability. During
skin grafting, the patient will lose approximately 1 ml of blood for each square
centimeter of skin harvested. During replacement of blood product losses for
tangential excisions, calcium must be administered along with any citrate-containing
blood products (including especially FFP and platelets), since burn patients tend to be
hypocalcemic even prior to transfusion.
TWolfe
5/99
LATEX PRECAUTIONS
History: In 1979, Nutter described the first cutaneous reaction to latex, a natural
rubber compound composed of over 200 proteins. In the late 1980's, reports of
intraoperative anaphylaxis due to latex began to appear in the literature. Children with
spina bifida and congenital urologic anomalies were identified as high risk, since these
patients were exposed to latex repeatedly for bladder catheterization and multiple
surgical procedures. In recent years, prevention (ie, avoiding latex exposure) has become
the standard of care at Riley and elsewhere.
Reactions Associated with Latex:
Latex sensitization refers to the presence of IgE antibodies to latex. One a few who are
latex-sensitized will have an actual allergic response to latex. There are 3 common
reactions to latex:
1. Irritant contact dermatitis. This is a usually benign, non-IgE-mediated skin
reaction, often due to the alkaline pH of the powdered gloves, and not a marker of
anaphylaxis risk.
2. Allergic contact dermatitis (Type IV cell-mediated hypersensitivity). This is a
delayed onset reaction, not life-threatening, beginning 2 or 3 days after exposure, and
can lead to erythema with vesicles.
3. Type I IgE-mediated hypersensitivity. This is the reaction that, upon re-exposure to
the allergen, can lead to immediate degranulation of mast cels and basophils, and produce
reactions of concern to the anesthesiologist, ranging from local urticaria to anaphylaxis.
Latex Precautions are used for at-risk individuals, which includes all patients with
myelomeningocele and some others with neurologic or urologic abnormalities.
History: Has patient ever had an anaphylactic reaction to latex? Also inquire about
urticaria or hives that have occurred within minutes of contacting bandages, balloons
or other latex-containing products.
Currently, premedication with H1 (diphenylhydramine) or H2 blockers (ranitidine) or
steroids is not recommended for patients with known latex allergy.
Notify OR nursing personnel. They will post sign on OR door, and make sure that latexcontaining products and gloves are avoided.
Notify anesthesia nurse. She will make latex-free gloves readily available for us, and
put the latex-containing gloves where they are less accessible. She will provide a
latex-free breathing bag, and if desired a latex-free oxygen SAT probe. Some of us use
the latex-containing probe, since it is more reliable and since anaphylactic reactions
to latex have not been reported secondary to cutaneous exposure from monitoring
devices.
IV tubing and ports: Our current IV tubing has all latex-free ports. All stopcocks
are safe ports.
Drugs and syringes: Most syringes are latex-free, and so labeled. Many stoppers are
made of neoprenex-free tourniqueLatex-free ports are identified as such on IV tubing.
In high-risk patients (ie, those who have had a known anaphylactic reaction to latex)
do not inject through ports not identified as latex-free, as anaphylaxis has been
reported with this kind of lapse.
ETT's, LMA's and airways: Endotracheal tubes and LMA's are latex-free, as are oral
airways. However, some of the nasal airways contain latex.
In case of an Anaphylactic Reaction to Latex (or any allergen)
1. Discontinue contact with the potential trigger, and reduce/stop anesthetic.
2. Stop any recently started infusion of drug or blood products.
3. 100% oxygen, volume resuscitation.
4. Pharmacologic treatment
a. The most specific medication is epinephrine. Epinephrine’s alpha properties
sustain blood pressure, and it’s beta-2 properties relieve bronchoconstriction.
b. Bronchodilators: albuterol and ipratropium via nebulizers
c. H2 blocks: ranitidine 150 mg or cimetidine 400 mg IV bolus
d. steroids: dexamethasone, 0.5 mg/kg
5. When the patient is stable, send a serum B-tryptase level. B-tryptase is a protease
stored in mast-cell granuales, and released during anaphylactic reactions. It’s serum
half-life is 30 minutes, but it may be eleveated for hours following an anaphylactic
reaction.
References:
Hepner DL, Castells MC:
2003;96:1219-29.
Updated 6/04
Latex allergy: An update.
Review Article in Anesth Analg
MALIGNANT HYPERTHERMIA
Definition: Malignant hyperthermia is an acute, potentially fatal pharmacogenetic disease
in which skeletal muscles increase their oxygen consumption and CO2 and lactate
production, resulting in heat production, respiratory and metabolic acidosis, muscle
rigidity, sympathetic stimulation, and increased cellular permeability. Incidence is
about 1:12,000 in children, 1:40,000 adults. MH is inherited as an autosomal dominant
trait. Potent inhaled agents, especially halothane, and also succinylcholine are specific
triggers for the disorder.
Diagnosis: MH is characterized by signs of hypermetabolism. In approximate order of
importance, early signs include:
masseter spasm following succinylcholine (variable)
unexplained tachycardia
elevated end-tidal CO2 (tachypnea in unparalyzed patient)
combined respiratory and metabolic acidosis
dysrhythmias
cyanosis
peripheral vasoconstriction (mottled skin) and profuse sweating
unstable blood pressure
hyperkalemia
hyperthermia as high as 42 C
myoglobinuria
elevated CPK (late)
Treatment: The only specific treatment of MH is dantrolene. In approximate order, call
for help and do the following:
Flush anesthesia from circuit and hyperventilate with 100% O2 circuit that contains no
triggering anesthetic
Give dantrolene in 1 mg/kg increments IV up to maximum of 10 mg/kg
Initiate cooling
Iced IV balanced salt solutions
Surface cooling with ice bags, cooling matress
Lavage stomach, bladder, rectum, peritneal cavity and/or thoracic cavity with
iced saline
Consider extracorporeal circulation with heat exchanger
Correct acidosis and hyperkalemia
Secure monitors, especially arterial line and Foley catheter
Maintain urine output of at least 2 ml/kg/hr
Consider mannitol up to 0.125 g/kg or furosemide 10 mg/kg
Consider 0.2 U/kg insulin with 50% D/W 1 ml/kg as IV bolus
ICU monitoring for a minimum of 24 hrs mandatory
Post-crisis followup therapy of oral dantrolene 1-2 mg/kg qid for 1-3 days
Labs to follow: ABG's, electrolytes esp K, myoglobinemia and myoglobinuria, CPK
MH-Susceptible Patients: In patients with a strong family history of MH, or who are
biopsy positive, or who have had a previous episode, observe the following:
Consider administering dantrolene prophylactically in a dose of 2.5 mg/kg IV
Avoid the potent inhaled anesthetics and succinylcholine
Use non-triggering agents, which include:
Barbiturates
Nitrous oxide
Propofol
Opiods
Non-depolarizing relaxants
Local anesthetics
Ketamine
Anticholinesterases
Anticholinergics
End-tidal CO2 monitoring absolutely essential from the onset of the case
Have dantrolene and other materials necessary for dealing with crisis readily available
Use an anesthetic machine that has been properly prepared:
Remove or disable (tape in “off” postion) all vaporizers
flush machine with oxygen @ 10L/min X 5 min
replace fresh gas outlet hose
use new disposable circuit
use new soda lime
overnight observation is not generally necessary if case was uneventful
Biopsies: The only definitive diagnosis available for MH consists of a caffeine-halothane
contracture test requiring 2 grams of fresh muscle. This can be performed in only a few
centers, notably the Mayo Clinic in the midwest. It is not recommended for small children
(less than 4 years of age or under 20 kg) because it requires a large amount of muscle,
and because controls in this age are lacking, making results difficult to interpret. In
older patients, the tests are 97% sensitive (3% false positives) and fairly specific (10%
false positives).
References:
Rosenberg H, et al: Testing for malignant hyperthermia.
2/02
TWolfe
Anesthesiology 2002;96:232-7.
PEDIATRIC RESUSCITATION
AGE
Preterm
Neonate
6 mo
1 yr
2 yr
4 yr
mm ID
3.0
3.0
3.5
4.0
4.5
5.0
ETT SIZE
cm depth
6 + kg wgt
9-10
11
12
13
14
DEFIBRILLATION
Wgt (kg)
Joules/kg
0-20
2
20-40
4
40+
6
(reduce dose for cardioversion)
Wgt (kg)
0.5-3
3-4
7
10
only
18
NORMAL VALUES
HR
150
140
135
130
110
100
BP
50/30
60/40
85/60
90/60
95/60
95/65
CARDIAC MASSAGE
Age
Compressions/min
Neonate
120
Infant
100
Child
80-100
(ventilation to compression ratio 1:5)
Drug
Epinephrine 1:10,000
Phenylephrine
Sodium Bicarbonate
Calcium gluconate
Lidocaine
Atropine
FIRST LINE DRUGS
Dose
10 ug/kg (0.1 ml/kg)
10 ug/kg
(BD X kg X 0.3) / 2
30-60 mg/kg
1 mg/kg
0.02 mg/kg (max 1 mg)
Comments
and
only
Give slowly; ppt with Ca
May cause bradycardia
Max 3-4 mg/kg/hr
Not useful for hypoxic brady
Drug
Adenosine
Dantrolene
Ephedrine
Esmolol
Mannitol
Propanolol
OTHER USEFUL DRUGS
Dose
50-200 ug/kg, rapid push
1-2 mg/kg (max 10 mg/kg)
0.1 mg/kg
500 ug/kg
0.25 - 1.0 gm/kg
0.05 - 0.1 mg/kg
Comments
For PSVT
For malignant hyperthermia
Predominantly b; indirect acting
For SVT, hypertension
Reduce ICP; osmotic diuretic
For TOF, IHSS
Drug
Amiodarone
Dopamine
Dobutamine
Epinephrine
"Epi - Cal"
Esmolol
Isoproterenol
Lidocaine
Milrinone
Nitroglycerin
Nitroprusside
PGE1
Tolazoline
Vasopressin
STANDARD INFUSIONS
Standard Mixture
450 mg / 250 ml
400 mg / 250 ml
500 mg / 250 ml
4 mg / 250 ml
Epi plus 1 gm Ca gluconate
5 gm / 500
2 mg / 250 ml
2 grams / 500 ml
20 mg / 100 ml premix
50 mg / 250 ml
50 mg / 250 ml
1 mg / 100 ml
100 mg / 100 ml
100 U / 250 ml
Dose Range
5 – 10 ug/kg/min
2-20 ug/kg/min
5-20 ug/kg/min
10-100+ ug/kg/min
Same as epi drip
50-200 ug/kg/min
10 - 100+ ug/kg/min
20-50 ug/kg/min
Load 50 ug/kg, 0.5 ug/kg/min
0.5-10 ug/kg/min
0.5-10 ug/kg/min
0.1 ug/kg/min
16-33 ug/kg/min
0.3 – 2 milliunits/kg/min