Cardiac Puncture SOP: Rodent Blood Collection
advertisement

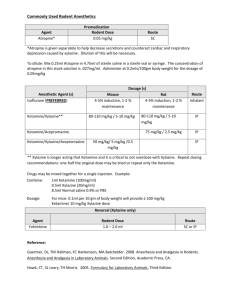
Griffith University Animal Ethics Manual- Laboratory STANDARD OPERATING PROCEDURES SOP No: L-6 SUBJECT: Cardiac Puncture to Collect Blood from Rodents (Non Recovery) POLICY: The Code (1) specifies; 3.3.13 Animals must be handled only be personnel instructed and competent in methods that avoid pain or distress. 3.3.32 For non-recovery surgery, the animal must remain unconscious throughout the procedure. PRECAUTIONS: Personnel conducting this procedure must have been vaccinated against Tetanus and Hepatitis B and should wear Personal Protective Equipment (Lab coat/gown, gloves, eye protection and masks). EQUIPMENT: 26 gauge ½ inch needle and 1 cc syringe. Ketamine (100mg/ml), Xylazine (20mg/ml) and 1.5ml Eppendorf tube. Micro-centrifuge. PROCEDURE: 1. Procedure is to take place in a quiet, clean environment, separate from other animals. 2. Draw required dosages of anesthetic solutions into the syringe. Mouse (50mgs/kg Ketamine and 10mgs/kg Xylazine). Rat (50mgs/kg Ketamine and 10mgs/kg Xylazine). 3. Injecting dosage intra-peritoneally. a. Restrain animal securely at the scruff with its head lower than body to avoid injury to organs during injection. b. Swab lower right quadrant of abdomen with alcohol. c. Introduce needle slowly through skin, subcutaneous tissue and abdominal wall. 4. Withdrawal plunger marginally to check needle is not in bladder or intestines. If no fluid is withdrawn begin to slowly inject dosage. 5. Place animal into cage free from structural obstacles and cover with towel to create a quiet, dim environment. 6. Monitor animal closely until signs of 2nd stage anesthesia occurs. Loss of consciousness may involve involuntary movement and breath holding. 7. Remove animal from cage monitor until 3rd stage anesthesia occurs. Confirm anesthetic depth, overall body muscle relaxation and absence of; Pedal or Withdrawal reflex, Palpebral reflex and Jaw tone reflex. 8. Place animal in a dorsal recumbency position on a clean and sterile surface and swab injection site with alcohol. Collecting blood from a terminally anesthetized rat. 9. Using a sterile syringe insert needle just behind the xiphoid cartilage and slightly left of the midline (as above). Advance needle at a 10-30 degree angle from the horizontal axis of the sternum, aspirating lightly while advancing. 10. Slowly withdraw blood until required sample is obtained; Mouse: ≥1ml, Rat: ≥10ml. 11. Humanely euthanize animal via cervical dislocation immediately after blood collection. Death must be established by lack of responses (pedal/withdrawal reflex, palpebral reflex, body relaxation) and loss of pulse before disposal of the carcass. 12. Transfer whole blood sample to a 1.5ml Eppendorf tube. 13. If serum sample is required allow blood to clot at ambient temperature for a minimum of 30 minutes, refrigerate at 4C for 1 hour to allow clot to contract. Centrifuge on low speed for 510 minutes and then extract serum with a sterile pipette and transfer to sterile 1.5ml Eppendorf tube. RECOMMENDATIONS: The procedure must be conducted by technicians previously certified competent in small animal care, restraint, IP injections and species specific anaesthesia / euthanasia techniques or, under the direct supervision of such a competent person. DATE ISSUED: REVISED: May 2008 2011 Dr G Harrison CHAIR OF GUAEC REFERENCES 1. NHMRC (2004) Australian code of practice for the care and use of animals for scientific purposes (7th edition). Australian Government. 2. http://www.animalethics.org.au/reader/research-procedures/arrp-blood-collection.htm 3. http://www.lal.org.uk/pdffiles/BLOOD.PDF 4. http://www.jhu.edu/animalcare/Mouse.HTM#blood 5. http://www.jhu.edu/animalcare/rat.htm#blood