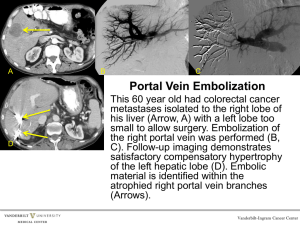
- e
advertisement

TIPS Sonography – Updated (Transjugular Intrahepatic Portosystemic Shunts) Author: Sharlene A. Teefey, M.D. Objectives: Upon the completion of this CME article, the reader will be able to: 1. Discuss the types of patients in which a TIPS stent might be placed, the benefits and contraindications, and the reasons behind why the stent might malfunction. 2. Describe the sonographic techniques that can be used to best visualize the vessels involved in hepatic flow. 3. Discuss the various sonographic parameters, such as stent velocity, main portal vein velocity, hepatic artery velocity and flow direction, that can be utilized when scanning a patient for the possibility of TIPS stent stenosis Background In patient populations with cirrhosis, the prevalence of esophageal varices ranges from 25 to 70% depending on the number of patients with end stage liver disease. Up to 33% of patients with documented varices will experience an episode of hemorrhage, usually within the first two years of diagnosis, and the risk for re-bleed approaches 70%. Despite aggressive medical management, the mortality rate from variceal hemorrhage is approximately 30% - 40%. Currently, endoscopic sclerotherapy or variceal band ligation are the accepted therapeutic interventions for acute variceal hemorrhage, although band ligation is preferred due to the reduced rate of re-bleeding and complications. The vasoactive drug, octreotide, may be used concomitantly. It decreases splanchnic arterial blood flow, which in turn, decreases portal venous blood flow and pressure. Up to 90% of variceal bleeding episodes can be controlled with endoscopic and/or pharmacologic therapy. For the 10% of patients who fail medical therapy, transjugular intrahepatic portosystemic shunt (TIPS) placement is recommended. Recurrent variceal hemorrhage is the most common indication for TIPS placement. Other indications include refractory ascites or hydrothorax related to chronic liver disease and Budd-Chiari syndrome. Improved control of ascites has been reported in 75% - 90% of patients. However, in patients with refractory ascites and advanced liver failure, there is a reported 42% early mortality rate when a stent is placed. There are several contraindications to TIPS placement. Patients with severe hepatic encephalopathy and liver failure are at high risk for worsening of their encephalopathy and liver function post TIPS and probably should not be stented. Likewise, patients with chronic portal vein thrombosis, in particular those with narrowed, sclerosed veins or cavernous transformation, are not candidates for TIPS placement. Nevertheless, experienced centers have been successful in placing a shunt in patients with subacute or acute thrombus. Severe right heart failure with an elevated central venous pressure is also a contraindication to TIPS placement. Relative contraindications include polycystic liver disease, systemic or hepatic infection and hypervascular liver neoplasms. In the past, uncovered stents have been used to treat patients with recurrent variceal bleeds. However, recently, covered stents have become available. These stents are covered with a polytetrafluoroethylene (PTFE) material. This material is anti-thrombogenic and it prevents pseudointimal proliferation. It is important however to place the covered portion of the stent along the entire parenchymal tract from the portal vein to the inferior vena cava to prevent hepatic vein stenosis. When comparing uncovered and covered stents, there are many advantages to the covered stents. While the immediate technical success rate of placement of both types of stents is 90% - 100%, the primary patency rate at 1 year (time to first intervention) ranges between 23% - 66% for the uncovered stents and 80% for the covered stents. In addition, the rate of recurrent variceal bleed averages 28% for the uncovered stents and 2.3% for the covered stents. There is also some question as to improved patient survival using the covered stents. TIPS placement is successful in controlling variceal bleeding in approximately 90% of patients. In those patients in whom a TIPS has been created but who experience recurrent variceal bleeding, most have evidence of stent malfunction (thrombosis, stent retraction into the hepatic parenchyma, or stenosis). Early TIPS occlusion (within the first two to three weeks) usually results from thrombosis (figure 1). Stent malfunction occurring after 3 weeks time is thought to be due to pseudointimal hyperplasia from bile extravasation; however other studies have suggested that stent stenosis may be due to a fibrotic response from shunt placement. Although venography is the gold standard for detecting stent malfunction, it is invasive and not an ideal screening test in the asymptomatic patient. Thus, several centers have turned to duplex and color Doppler sonography as a means to evaluate and follow patients with TIPS. Sonographic Technique Because most TIPS are placed deeply within the liver, usually between the right or middle hepatic vein and right portal vein, a low frequency transducer is required for optimal penetration. Given the high velocity flow through the shunt, artifact due to aliasing can also be minimized or eliminated using a lower frequency transducer. At our institution, we use a transducer with a center frequency of 2.5 MHz. While scanning the stent, the Doppler scale should be adjusted to reflect the very high velocities within it. The scale should be increased until homogeneous color opacification is demonstrated. This will allow the detection of focal areas of color aliasing suggestive of a stenosis. When determining peak systolic velocity, it is important to obtain an angle corrected waveform at 60° or less. Several approaches can be used. The distal (portal venous) portion of the stent is usually best imaged from a high anterolateral intercostal or subcostal/subxyphoid approach. It is important when obtaining a waveform to place the cursor beyond the right portal vein and within the intraparenchymal portion of the stent so as not to obtain an artifactually low peak systolic velocity that reflects portal vein velocity rather than true stent velocity. The proximal (hepatic vein) portion of the stent and hepatic vein are often best seen from a low intercostal or subcostal/subxyphoid approach. When evaluating the intrahepatic portions of the right and left portal vein branches, the main portal vein, and peripheral portion of the draining hepatic vein, the Doppler scale should be decreased. The right portal vein and main portal vein are best imaged through an intercostal approach. The left portal vein can be imaged sagittally in the subxyphoid region. The abdomen and pelvis should also be scanned to determine if there has been an increase or decrease in ascites, in particular in those patients in whom the stent was placed for control of ascites refractory to conventional therapy. It is also important to search for collaterals that would further suggest shunt malfunction. Sonographic Parameters for the Detection of Stent Stenosis A large number of studies have been published which have suggested various Doppler parameters that can be used to diagnose shunt malfunction. These include peak systolic velocity within the stent, the difference between the maximum and minimum velocity in the stent, temporal change in the stent velocity, velocity in the main portal vein, main portal vein and stent pulsatility, flow direction in the right and left portal vein branches, peak systolic velocity in the hepatic artery, and flow direction in the draining hepatic vein. Although most studies suggest that Doppler sonography is accurate in identifying shunt malfunction, many of the parameters used to differentiate a patent from a stenotic shunt are not uniformly agreed upon. Furthermore, the angiographic criteria used to diagnose stent stenosis vary from institution to institution. Stent Velocity Stent stenoses can be diffuse, with narrowing throughout the stent, or focal. Focal stenoses are much more common. While several studies have found that most focal stenoses occur in the hepatic vein at the stent entry site, at our institution, we found a nearly equal distribution of stenoses in the shunt and in the hepatic vein at the stent entry site. Interestingly, Saxon et al recently reported that patients with shunt stenoses were much more likely to experience recurrent variceal bleeding than those with hepatic vein stenoses. Stent velocity is the most common parameter that has been used to diagnose a stenosis. Several centers have reported 50 to 60 cm/sec as the lower limit of normal for stent velocity. Values below this level would reflect the expected decrease in velocity proximal or distal to a stenosis. However, these studies are limited by the small number of stent stenoses reported (in part because many of the sonograms were obtained in the immediate post procedure period), the lack of a uniform angiographic definition of stent stenosis (it was not defined in one study and differed in the other two), and the use of different site(s) of velocity measurement (in one study some distal stent velocities may have been obtained from the portion of the stent that projected into the portal vein, artifactually lowering values ). Dodd, et al, failed to detect a stenosis in 14 of 15 cases when applying a stent threshold of 50 to 60 cm/sec. While Feldstein, et al, reported a specificity of 99% and sensitivity of 78% using 50cm/sec as the lower limit of normal in 32 stenotic shunts. Using this same value, we reported a similarly high specificity of 88% but a sensitivity of only 32% in 34 stenotic shunts. Likewise, when Haskal, et al, and Murphy, et al, applied the value of <60 cm/sec to their patient populations, their specificities for shunt malfunction were 89% and 93% but their sensitivities were only 57% and 25%, respectively. While it appears that this threshold value is very specific for stent stenosis, it is at the expense of missing many less severe stenoses. Although the mortality rate from recurrent variceal hemorrhage in TIPS patients is probably lower than in non-TIPS patients, if ultrasound is to be used as a screening test, ideally it should have a high sensitivity for the detection of shunt malfunction. However, this must be balanced against the resultant lowered specificity and increased risk and cost of performing unnecessary angiographic studies. Our data suggest that 90 cm/sec is a more appropriate lower limit of normal. When Feldstein, et al, used this value as a lower limit of normal, their sensitivity increased to 93% and specificity decreased to 55% for detecting stent stenoses. While this value allows us to detect less severe stenoses earlier than might otherwise be detected by using a lower threshold, the increased number of sonographic studies and/or venograms is not unacceptably high. Several centers have also determined the upper limit of normal for stent velocity. Values above this level would reflect the expected increase in velocity at the site of a stenosis. The reported upper limit of normal ranged from 185 to 220 cm/sec. We reported a similar value of 190 cm/sec. When we combined the upper and lower limits of normal to produce a velocity range of 90 to 190 cm/sec, we achieved a sensitivity of 82% and specificity of 72% for detecting shunt malfunction. Because the velocity through a stenosis increases (figure 2) and proximal to a stenosis decreases, the difference between the two (velocity gradient) should suggest in the presence of a stenosis. Our data from the 25 most recent cases in which we correlated velocity gradient with the absence or presence of a stenosis showed that a gradient of >100 cm/sec has a positive predictive value of 82% for a stenosis. However, our sensitivity was only 56% indicating that many patients with stenoses do not have abnormal velocity gradients. Although uncommon, a diffuse stenosis may account for some of the cases. Temporal differences in peak stent velocity have also been evaluated in an attempt to diagnose stent stenoses. If a stent proximal to a stenosis were evaluated, a temporal decrease in velocity would be expected if a stenosis had developed; whereas, if the stenotic segment itself were evaluated, the velocity would increase over time. Dodd, et al, reported that an increase or decrease of >50 cm/sec from the post-TIPS baseline sonogram resulted in a sensitivity of 93% and specificity of 77% for the detection of stent stenoses. Our results were somewhat similar to Dodd’s – a decrease of 40 cm/sec or increase of 60 cm/sec had a sensitivity of 75% and specificity of 84%. Main Portal Vein Velocity Main portal vein velocity increases after placement of a TIPS because the stent serves as a low resistance conduit and bypasses the high resistance hepatic circulation. The reported mean main portal vein velocity in patients with patent shunts ranged from 37 to 47 cm/sec. Our reported value of 43 cm/sec falls within this range. A decrease in main portal vein velocity suggests a stent stenosis or occlusion. The reported mean main portal vein velocity in patients with compromised shunts ranged from 31 to 33 cm/sec. In fact, in the study by Murphy, et al, the best predictor for determining shunt stenosis was main portal vein velocity. Our data showed a similar value of 30 cm/sec as the lower limit of normal for main portal vein velocity with a sensitivity of 82% and specificity of 77%. Shunt and Main Portal Vein Pulsatility It has recently been shown that pulsatile flow is present immediately after TIPS placement and declines in patients with TIPS malfunction. Pulsatile flow in the main portal vein and shunt is secondary to transmitted cardiac pulsations and can be measured by calculating a venous pulsatility index (V max - V min / V max). Measurements should be obtained in the main portal vein and proximal, mid, and distal aspects of the shunt during quiet respiration. The authors found that a venous pulsatility index < 0.16 was 94% sensitive and 87% specific for shunt malfunction. Portal Vein Branch Flow Direction After placement of a TIPS, flow direction in the right and left portal vein branches reverses from hepatopetal to hepatofugal (i.e. towards the shunt) in most patients because of the decreased resistance to flow provided by the shunt. However, if the shunt becomes occluded or stenosed, it can no longer serve as a low resistance conduit and flow direction in the portal vein branches may again change from hepatofugal to hepatopetal (figure 3). This finding was reported in a limited number of patients and was indicative of stent stenosis or occlusion. In our study, change in portal vein branch flow direction (from hepatopetal to hepatofugal) had a specificity of 83% and positive predictive value of 86%, but a sensitivity of only 15% - 31%. Based on our most recent experience, it appears that change in portal vein branch flow direction is a late sign of stent malfunction. Hepatic Artery Velocity Following TIPS placement, there is a compensatory increase in hepatic artery flow because of the diversion of portal vein flow into the newly created low resistance conduit that bypasses the liver. Foshager, et al, have shown an increase in hepatic artery peak systolic velocity from 79 cm/sec prior to TIPS placement to 131 cm/sec one day after TIPS placement. Although we found no statistically significant difference in hepatic artery velocity or resistive index in patients with patent and stenotic stents, Haskal, et al, reported a significant decline in hepatic artery velocity from 135 cm/sec to 108 cm/sec in patients with shunt compromise. Hepatic Vein Flow Direction When a stenosis develops in the stent proximal to where it exits the hepatic vein or in the hepatic vein itself (between the shunt and inferior vena cava) (figure 4), flow in the hepatic vein distal to the shunt may be reversed (figure 5), that is, hepatopetal. This finding has been reported, but its sensitivity is unknown. Combining Parameters Although some centers have reported that combining velocity parameters did not improve their accuracy in predicting shunt stenosis, when we included in our analysis the overall impression of the radiologist performing the sonographic examination, which was based on multiple parameters as outlined in the Table, our sensitivity was 92% and specificity 72%. Additional parameters not listed in the Table (such as reversal of flow in the right and left portal vein branches and draining hepatic vein) were also used in formulating an overall impression. Conclusion From the above discussion, it is evident that many different parameters have been studied in an attempt to determine which are the most accurate for detecting stent malfunction. However, direct comparison of these parameters is difficult because the ultrasound protocols varied from institution to institution, velocity measurements were obtained from one or more different sites in the stent, and different sonographic parameters were analyzed (peak systolic stent velocity versus temporal change in stent velocity). There has also been little mention of intra or interobserver variability in obtaining these measurements. Furthermore, the angiographic definition of a hemodynamically significant shunt stenosis differed between centers. Until it is better understood which angiographic definition of shunt malfunction (elevated portosystemic gradient versus percent anatomic stenosis) most accurately reflects the redevelopment of portal hypertension (and an increased risk for a re-bleed) in the post-TIPS patient, it will be difficult to determine which sonographic parameters are most accurate in predicting a hemodynamically significant shunt stenosis. With the advent of covered stents, comparative studies are now needed to compare covered and uncovered stents to evaluate re-stenosis rates, mortality and survival rates, and encephalopathy rates. Table: Mallinckrodt Data – Suggested Doppler Criteria for TIPS Malfunction Doppler Parameter Vel. (cm/sec) Sensitivity Specificity PPV NPV Peak Shunt Velocity < 90 or > 190 84% 70% 82% 72% Change in Peak Shunt Decrease > 40 or 71% 88% 89% 67% Velocity Increase > 60 Main Portal Vein Velocity < 30 82% 77% 86% 71% Overall Impression Not Applicable 92% 72% 84% 86% Figures: 1 Occluded TIPS 2 Mid stent stenosis with increased velocity through the stenosis 3 Stent stenosis with flow reversal in the RPV 4 Hepatic vein stenosis using color Doppler 5 Distal stent stenosis with flow reversal in the draining hepatic vein References or Suggested Reading: 1. Roberts LR, Kamath PS. Pathophysiology and treatment of variceal hemorrhage. Mayo Clin Proc 1996; 71:973-983. 2. Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. Am J Gastroenterol 1997; 92:1081-1091. 3. Sanyal AJ, Freedman AM, Luketic VA, et al. Transjugular intrahepatic portosystemic shunts for patients with active variceal hemorrhage unresponsive to sclerotherapy. Gastroenterology 1996; 111:138-146. 4. Brown RS, Jr., Lake JR. Transjugular intrahepatic portosystemic shunt as a form of treatment for portal hypertension: indications and contraindications. In: Schrier RW, ed. Advances in Internal Medicine. St. Louis: Mosby, 1997; 42:485-504. 5. Ferral H, Gambosa P, Postoak DW, et al. Survival after elective Transjugular intrahepatic portosystemic shunt creation: prediction with model for end-stage liver disease score. Radiology 2004; 231:231-236. 6. Kerlan RK, Jr., LaBerge JM, Gordon RL, Ring EJ. Transjugular intrahepatic portosystemic shunts: current status. AJR 1995; 164:1059-1066. 7. Hausegger KA, Karnel F, Georgieva B, et al. Transjugular intrahepatic portosystemic shunt creation with the Viatorr expanded polytetrafluoroethylene-covered stentgraft.J Vasc Interv Radiol. 2004;15:239-48. 8. Vignali C, Bargellini I, Grosso M, et al. TIPS with expanded polytetrafluoroethylenecovered stent: results of an Italian multicenter study. AJR 2005;185:472-80. 9. Teng GJ, Lu Q. Bile leakage during transjugular intrahepatic portosystemic shunt creation: in vitro effect of bile on growth and function of human umbilical vein endothelium. Radiology 2005; 235:867-871. 10. Saxon RR, Ross PL, Mendel-Hartvig J, et al. Transjugular intrahepatic portosystemic shunt patency and the importance of stenosis location in the development of recurrent symptoms. Radiology 1998; 207:683-693. 11. Haskal ZJ, Pentecost MJ, Soulen MC, et al. Transjugular intrahepatic portosystemic shunt stenosis and revision: early and midterm results. AJR 1994; 163:439-444. 12. Rössle M, Haag K, Ochs A, et al. The transjugular intrahepatic portosystemic stentshunt procedure for variceal bleeding. N Engl J Med 1994; 330:165-171. 13. Kanterman RY, Darcy MD, Middleton WD, Sterling KM, Teefey SA, Pilgram TK. Doppler sonography findings associated with transjugular intrahepatic portosystemic shunt malfunction. AJR 1997; 168:467-472. 14. Chong WK, Malisch TA, Mazer MJ, et al. Transjugular intrahepatic portosystemic shunt: US assessment with maximum flow velocity. Radiology 1993; 189:789-793. 15. Foshager MC, Ferral H, Nazarian GK, et al. Duplex sonography after transjugular intrahepatic portosystemic shunts (TIPS): normal hemodynamic findings and efficacy in predicting shunt patency and stenosis. AJR 1995; 165:1-7. 16. Feldstein VA, Patel MD, LaBerge JM. Transjugular intrahepatic portosystemic shunts: accuracy of Doppler US in determination of patency and detection of stenoses. Radiology 1996; 201:141-147. 17. Dodd GD, III, Zajko AB, Orons PD, et al. Detection of transjugular intrahepatic portosystemic shunt dysfunction: value of duplex Doppler sonography. AJR 1995; 164:1119-1124. 18. Haskal ZJ, Carroll JW, Jacobs JE, et al. Sonography of transjugular intrahepatic portosystemic shunts: detection of elevated portosystemic gradients and loss of shunt function. JVIR 1997; 8:549-556. 19. Murphy TP, Beecham RP, Kim HM, Webb MS, Scola F. Long-term follow-up after TIPS: use of Doppler velocity criteria for detecting elevation of the portosystemic gradient. JVIR 1998; 9:275-281. 20. Surratt RS, Middleton WD, Darcy MD, Melson GL, Brink JA.. Morphologic and hemodynamic findings at sonography before and after creation of a transjugular intrahepatic portosystemic shunt. AJR 1993; 160:627-630. 21. Longo JM, Bilbao JI, Rousseau HP, et al. Transjugular intrahepatic portosystemic shunt: evaluation with Doppler sonography. Radiology 1993; 186:529-534. 22. Sheiman RG, Vrachliotis T, Brophy DP, Ransil BJ. Transmitted cardiac pulsations as an indicator of transjugular intrahepatic portosystemic shunt function: initial observations. Radiology 2002; 224:2250230. 23. Feldstein VA, LaBerge JM. Hepatic vein flow reversal at duplex sonography: a sign of transjugular intrahepatic portosystemic shunt dysfunction. AJR 1994;162:839-41. About the Author: Sharlene A. Teefey, M.D. is currently an Associate Professor of Radiology at the Mallinckrodt Institute of Radiology at Washington University School of Medicine in St. Louis Missouri. She is a member of numerous societies and organizations including the American College of Radiology, the Society of Radiologists in Ultrasound, and the American Institute of Ultrasound in Medicine. She is a reviewer of manuscripts for Radiology, the American Journal of Roentgenology, and Radiographics. She has more than 45 publications in peer review medical journals and has been a speaker at numerous institutions and conferences across the country. Examination: 1. In those patients with cirrhosis that have developed esophageal varices, up to ____ will experience an episode of hemorrhage, usually within two years of the diagnosis. A. 5% B. 10% C. 25% D. 33% E. 45% 2. TIPS placement is recommended for the ______ of patients with esophageal varices who fail medical therapy. A. 1% B 5% C. 10% D. 25% E. 50% 3. The most common indication for TIPS placement is A. recurrent variceal hemorrhage B. hydrothorax related to liver disease C. patient preference D. refractory ascites E. Budd-Chiari Syndrome 4. There are several contraindications to TIPS placement, which include all of the following EXCEPT A. patients with severe hepatic encephalopathy B. patients with chronic portal vein thrombosis due to narrowed sclerosed veins C. patients with liver failure D. patients with chronic portal vein thrombosis due to cavernous transformation E. patients with severe left heart failure and an elevated systemic blood pressure 5. Relative contraindications to TIPS placement include all of the following EXCEPT A. recurrent variceal hemorrhage B. systemic infection C. hypervascular liver neoplasms D. polycystic liver disease E. hepatic infection 6. Regarding covered stents, all of the following statements are true EXCEPT A. The covering material helps prevent pseudointimal proliferation. B. The covering material is thrombogenic. C. It is important to place the covered portion of the stent along the entire parenchymal tract from the portal vein to the inferior vena cava. D. When comparing uncovered and covered stents, the primary patency rate for covered stents at 1 year appears to be higher. E. When comparing uncovered and covered stents, the rate of recurrent variceal bleed appears to be lower for the covered stents. 7. TIPS placement is successful in controlling variceal bleeding in approximately ____ of patients. A. 15% B. 33% C. 50% D. 67% E. 90% 8. In those patients in whom a TIPS has been created, early TIPS occlusion (within the first two to three weeks) is usually the result of A. pseudointimal hyperplasia B. stent retraction into the hepatic parenchyma C. stenosis D. thrombosis E. a fibrotic response 9. The gold standard for detecting stent malfunction is A. duplex and color Doppler B. MRA C. venography D. CT E. arteriography 10. Most TIPS are placed deep within the liver, usually between the A. right or middle hepatic vein and the right portal vein B. right or middle hepatic vein and the left hepatic vein C. left hepatic vein and the right hepatic artery vein D. right or middle hepatic artery and the left portal vein E. left hepatic artery and the right or middle hepatic vein 11. When determining peak systolic velocity, it is important to obtain an angle corrected waveform at A. 90° or less B. 60° or less C. 30° or less D. 60° or more E. 90° or more 12. Regarding sonographic technique, when evaluating the intrahepatic portions of the right and left portal vein branches, the main portal vein, and peripheral portion of the draining hepatic vein, the A. Doppler scale should be increased B. gain should be increased C. power should be increased D. gain should be decreased E. Doppler scale should be decreased 13. Some of the various Doppler parameters that can be used to diagnose shunt malfunction include all of the following EXCEPT A. peak systolic velocity within the stent B. hepatic vein pulsatility C. temporal change in the stent velocity D. velocity in the main portal vein E. the difference between the maximum and minimum velocity in the stent 14. _____ is the most common parameter that has been used to diagnose a stenosis. A. Stent velocity B. Main portal vein velocity C. Portal vein branch flow direction D. Hepatic artery velocity E. Hepatic vein flow direction 15. When evaluating a patient who has a TIPS utilizing “stent velocity”, A. only lower limits of normal have been suggested B. only upper limits of normal have been suggested C. both upper limits and lower limits of normal have been suggested D. temporal differences should be used not upper and lower limits E. velocity gradient should be used not upper and lower limits 16. “Velocity gradient” is the difference in velocity flow between the A. velocity distal to a stenosis and the velocity proximal to a stenosis B. velocity proximal to a stenosis and the velocity through a stenosis C. velocity through the portal vein and the velocity through a stenosis D. velocity proximal to a stenosis and the velocity through the portal vein E. velocity through the portal vein and the velocity distal to a stenosis 17. Regarding main portal vein velocity, after placement of a TIPS that is functioning, the velocity ____ because the stent serves as a low resistance conduit and bypasses the high resistance hepatic circulation. A. is pulsatile B. decreases C. remains the same D. increases E. increases briefly, then decreases 18. After TIPS placement, pulsatile flow in the main portal vein and shunt is secondary to A. increased flow in the hepatic artery B. increased flow in the hepatic vein C. transmitted cardiac pulsations D. reverberation within the stent E. increased flow in the inferior vena cava 19. Regarding portal vein branch flow direction, after placement of a TIPS in most patients, flow direction in the right and left portal vein branches A. increases from hepatofugal to hepatopetal B. reverses from hepatofugal to hepatopetal C. increases from hepatopetal to hepatofugal D. decreases from hepatopetal to hepatofugal E. reverses from hepatopetal to hepatofugal 20. Regarding hepatic vein flow direction, when a stenosis develops in the stent proximal to where it exits the hepatic vein or in the hepatic vein itself, flow in the hepatic vein distal to the shunt A. may be pulsatile B. may be decreased C. may be increased D may be reversed E. is unaffected