POSTOPERATIVE PAIN MANAGEMENT
advertisement

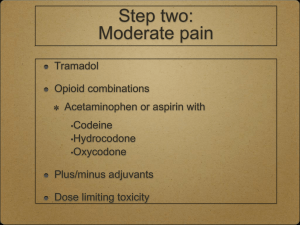
PEDIATRIC POSTOPERATIVE PAIN MANAGEMENT Reading Materials 1. UIC Medical Center Clinical Care Guideline for Pain Assessment and Management http://www.hospital.uic.edu/ [search under UI Medical Center Clinical Care Guidelines Pain] 2. University of Michigan Pain Management Website http://www.med.umich.edu/pain/pediatric.htm Patient Controlled Analgesia: With or Without a Basal Rate? Factors that influence the inclusion of basal infusion in patient-controlled analgesia A. Factors that bias toward inclusion of a basal infusion 1. Long-standing opioid use 2. Chronic illnesses, e.g. cancer and sickle cell disease 3. High pain severity 4. Very extensive surgery, especially for the first postoperative night 5. A history of inadequate analgesia with previous parameter choices 6. Depressed mood, anxiety disorders, or other emotional factors that would decrease the patient’s patience or ability to cope with delay in attaining relief B. Factors that bias against inclusion of a basal infusion 1. Significant airway, neurologic, or respiratory compromise 2. Relatively mild pain 3. No previous exposure to opioids 4. Surgery that could result in bleeding into the airway. e.g. maxillofacial procedures, alveolar bone grafting, pharyngeal flaps 5. Not very extensive surgery 6. More than 1 to 2 days postoperatively 7. A history of somnolence or hypoventilation with previous opioid administration (Table 27.6, p.476. Pain in Infants, Children and Adolescents by Schechter, Berde, and Yaster. 2002) Transition to Oral Analgesics PCA Oral 1. Change to demand only. No basal infusion for a brief period. 2. Consider a co-analgesic while on the demand only dose. a. Acetaminophen: 10-15 mg/kg po q 4 hours b. Ibuprofen: 10 mg/kg q 6 hours c. Keterolac 0.5 mg/kg IV q 6 hours 3. Consider rescue IV medications a. Morphine b. Nalbuphine Oral Medications 1. Oxycodone 0.1 – 0.15 mg/kg po q 4 hours a. with acetaminophen or ibuprofen 2. Hydrocodone a. with acetaminophen or ibuprofen (Taken from Anesthesioogy Clinics of North America. 2005;23(4):780-814) (Berde CB, Sethna NF. Analgesics for the Treatment of Pain in Children. N Engl J Med 347:1094, October 3, 2002) APPROXIMATE OPIOID EQUIANALGESIC DOSES (PEDIATRIC)*** DRUG DOSE (mg) Parenteral DOSE (mg) Oral DURATION (hour) Morphine 1 3a 4 -6 Morphine – Sustained Release --- 3 8 –12 --- 3 24 Hydromorphone (Dilaudid) 0.15 0.75 4-5 Meperidine (Demerol) b 7.5 30 2-4 Fentanyl (Sublimaze) 0.01 (MS Contin, Oramorph SR) Morphine – Sustained Release (Kadian) 1 Codeine c (Tylenol with codeine #2, #3 or #4) 12 20 4-6 Hydrocodone d (Vicodin, Lortab) --- 3 4-6 Oxycodone e --- 3 4-6 --- 3 8 - 12 0.3 4–8 (Percodan, Percocet, Tylox, Roxicet, Roxicodone)) Oxycodone Controlled Release (Oxycontin) Methadone (Dolophine) f These following agents are NOT opioids. In general, they are weaker analgesics. The equianalgeisa dose is for information only and, with the exception of ketorolac, it is NOT a reasonable or appropriate dose. a b Ketorolac (Toradol) 1-3 1 6 Ibuprofen (Motrin) --- 130 6–8 Acetaminophen (Tylenol) --- 390 4 IM:PO ratio is 1:6 (1mg IM = 6mg po) with single or intermittent dosing. With chronic dosing, the ratio changes to 1:3 (1mg IM = 3mg PO). Not recommended for pain management. Keep doses under 600mg/day and do not use for > 48 hours. c Most preparations contain either 15, 30 or 60mg of codeine with 300mg acetaminophen. Do not exceed 75mg/kg/day or 2.6 grams/day in children under 12 years of age. Do not exceed 4 grams/day of acetaminophen in children > 12 years of age. d Preparations contain either 5, 7.5 or 10mg of hydrocodone in combination with acetaminophen (500, 650 or 750mg). Do not exceed 75mg/kg/day or 2.6 grams/day in children under 12 years of age. Do not exceed 4 grams/day of acetaminophen in children > 12 years of age. e Preparations contain 5mg oxycodone in combination with acetaminophen (325 or 500mg). Do not exceed 75mg/kg/day or 2.6 grams/day in children under 12 years of age. Do not exceed 4 grams/day of acetaminophen in children > 12 years of age. f Methadone is much more potent than previously described in the literature and it accumulates with repeated dosing. A highly individual and cautious approach when switching from another potent opioid must be taken. Please consult the Acute Pain Service. Equianalgesic Dose Conversion Formula *** Dose for current drug (from equianalgesic chart) = (scheduled and rescue doses) 24-hour dose of current drug Dose for new drug (from equianalgesic chart) = 24-hour dose of new drug * ** This table is 1/10 of the value of the equianalgesic doses for adults. These are NOT suggested starting doses; these are doses of opioids that produce approximately the same amount of analgesia. Published trials vary in the suggested doses that are equianalgesic to morphine. By using this table, you can determine a dose of a new opioid and/or route of administration that is approximately equal in analgesic effect to the dose of the former opioid. Titration to clinical response is necessary. Recommended doses do not apply to patients with renal or hepatic insufficiency or other conditions affecting drug metabolism and kinetics. Selected References: Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain, American Pain Society, 1999 Acute Pain Management in Adults: Operative Procedures. Quick Reference Guide for Clinicians. AHCPR Pub. No. 920019. 1992 Micromedex ® Healthcare Series Drugdex Drug Evaluations