Dr Asmat Salim MM707-electrophoresis 2014
advertisement

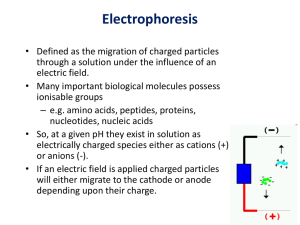
Core Course # MM 707 (Techniques in Molecular Medicine-II) Biochemistry Electrophoresis Western Blotting 1 Electrophoresis • Introduction • Theory and Basic Concepts • Types of Electrophoresis SDS-PAGE electrophoresis Native gel electrophoresis • Trouble shooting Introduction 3 Electrophoresis: The transport of particles through a solvent by an electric field is called electrophoresis. In the biological system, many molecules are electrically charged and will move if electric field is applied. In electrophoresis, macromolecules are characterized by their rate of movement in an electric field. This technique is used to (1) distinguish molecules on the basis of charge and shape (2) to determine molecular weight of proteins (3) to detect amino acid changes from charged to uncharged residues & (4) to separate different molecular species quantitatively. 4 Theory & Basic Concepts 5 What is happening during Electrophoresis: Some Basic Concepts • Separation of large (macro) molecules depends upon two forces: charge and mass. • When a biological sample, such as proteins or DNA, is mixed in a buffer solution and applied to a gel, these two forces act together. • The electrical current from one electrode repels the molecules while the other electrode simultaneously attracts the molecules. • The gel material acts as a "molecular sieve," separating the molecules by size. Molecules are forced to move through the pores when the electrical current is applied. 6 The rate of migration through the electric field depends on the (1) strength of the field (2) size and shape of the molecules, (3) on the ionic strength and temperature of the buffer in which the molecules are moving. 7 There is also a frictional resistance that slows down the movement of this charged molecule. This frictional force is a measure of the (1) hydrodynamic size of the molecule (2) the shape of the molecule (3) the pore size of the medium & (4) viscosity of the buffer 8 Mobility depends on charge Mobility depends on frictional coefficient which in turn depends on physical parameters of molecules Therefore, “mobility” gives information about the charge, size and shape of the molecule 9 • The current in the solution between the electrodes is conducted mainly by the buffer ions with a small proportion being conducted by the sample ions. • It is possible to accelerate an electrophoretic separation by increasing the applied voltage, which would result in a corresponding increase in the current flow. • The distance migrated will be proportional to current • However, increasing the voltage would result in the generation of heat. 10 Heating of the electrophoretic medium can have the following effects: 1. An increased rate of diffusion of sample and buffer ions leading to broadening of the separated samples. 2. Thermal instability of samples that are rather sensitive to heat. This may include denaturation of proteins or loss of activity of enzymes. 3. A decrease of buffer viscosity, and hence a reduction in the resistance of the medium. 11 Types of Electrophoresis 12 Types of Electrophoresis Moving Boundary Zone Paper Gel Polyacryalmide Non Dissociating (Native-PAGE) Agarose Dissociating (SDS-PAGE) 13 Gel electrophoresis * It is used for the separation of proteins and nucleic acids * Many types of gels are used as supporting medium e.g. starch, polyacrylamide and agarose * Earliest work in gel electrophoresis was done with starch * It provided the first evidence for the existence of isozymes. * Generally polyacrylamide gels are used for proteins and agarose for nucleic acids 14 Polyacrylamide Gel Electrophoresis 15 Polyacrylamide Gel Electrophoresis (PAGE) Gel Ingredients 1. Acrylamide 2. Bis acrylamide 3. Tris-HCl 4. N,N,N’,N’-tetramethylethelenediamine (TEMED) 5. Ammoinium per sulfate (APS) 16 Acrylamide, Bis Acrylamide & Polyacrylamide 17 Pore size 1. Pore size of polyacrylamide gel is dependent on the concentration of acrylamide 2. Pore size decreases as the conc. of acryalmide increases 3. High concentration of gels would be able to separate low molecular weight proteins 18 APS & TEMED Chemical and Photochemical • Polymerization is initiated by APS or riboflavin • Chemical: In TEMED-APS system, free base of TEMED catalyzes the formation of free radicals from persulphate and these in turn initiate polymerization. TEMED acts as the accelerator to polymerization process • Photochemical: Light is required for initiation of polymerization. Light causes photodecomposition of riboflavin and produce free radicals. • Oxygen inhibits polymerization 19 20 SDS-PAGE (Dissociating Buffer System) (Laemmli, 1970) The buffer system tends to dissociate all proteins into their individual polypeptide subunits. Most common dissociating agent is sodium dodecyl suphate, an anionic detergent. Before electrophoresis, samples are treated with a solution containing b-mercaptoethanol and SDS and heated at 1000C. b-mercaptoethanol is a reducing agent that cleaves disulfide bonds. SDS denatures proteins and coats proteins with negative charges, which overwhelm the protein’s intrinsic charge. Most polypeptides bind SDS at a constant ratio (i.e. 1.4 g per gram 21 polypeptide) Thus, treated proteins assume a rod-like shape and all carry the same net negative charge. When treated proteins are electrophoresed through a SDS-PAGE gel, proteins migrate toward the positive electrode. The proteins can now be separated by their molecular weights. 22 (a) (b) (c) 23 Native-PAGE (Non Dissociating Buffer System) • "Native" or "non-denaturing" gel electrophoresis is run in the absence of SDS. Since the protein retains its folded conformation, the mobility depends on both the protein's charge and its hydrodynamic size. • The electric charge driving the electrophoresis is governed by the intrinsic charge on the protein at the pH of the running buffer. This charge will, of course, depend on the amino acid composition of the protein as well as posttranslational modifications. • The higher mobility is for more compact conformations, & lower for larger structures. If native PAGE is carried out near neutral pH to avoid acid or alkaline denaturation, then it can be used to study conformation, selfassociation or aggregation, and the binding of other proteins or compounds. • Another advantage of native gels is that it is possible to recover proteins in their native state after the separation. Recovery of active biological materials may, however, need to be done prior to any fixing or staining 24 Continuous and Discontinuous Buffer Systems Continuous: Same buffer ions are present throughout the sample, gel and electrode reservoirs Sample is loaded directly on to the gel in which the separation will occur, called the separating or resolving gel which has pores sufficiently small to resolve the proteins Discontinuous: Different buffer ions are present in the gel and electrode reservoirs Sample is loaded on to large pore-size gel called the stacking gel polymerized on top of the resolving gel 25 Advantage: Large volumes of dilute samples can be applied and gives good resolution as proteins are concentrated into narrow zones (or stacks) during migration through the stacking gel prior to their separation in the resolving gel. 26 Electrophoresis Buffers Tris HCl pH 6.8 = Stacking gel Tris HCl pH 8.8 = Resolving gel Tris-glycine pH 8.3 = Running buffer Tris-HCl pH 6.8 & glycerol = Sample diluting buffer 27 28 Formation of an ion front: Cl = Leading ion Gly = Trailing ion 29 What happens to the proteins? Proteins have mobilities between those of Gly and Cl-. 30 In separating gel Glycine mobility increases, becomes greater than protein mobility, but still slower than Cl-. • Protein sample, now in a narrow band, encounters both the increase in pH and decrease in pore size. • Increase in pH would tend to increase electrophoretic mobility, but smaller pores decrease mobility. • Relative rate of movement of ions in lower gel is chloride > gly > protein. • Proteins separate based on charge/mass ratio and on size and shape parameters. 31 Slab gel Electrophoresis 32 Gel preparation and sample loading 33 34 Visualizing & Analyzing the Gel 35 Staining the Gels Normally the target molecules of the electrophoretic process must be visualized in a staining process after the separation. (1) Direct Staining → Coomassie brilliant blue R250 (2) Silver Staining → Silver Nitrate 36 Direct Staining → Coomassie brilliant blue • Normally Coomassie dye is used for staining proteins after SDS and native electrophoresis. • A commonly used stain for detecting proteins in polyacrylamide gels is 0.1% Coomassie Blue dye in 50% methanol, 10% glacial acetic acid. Acidified methanol precipitates the proteins • The dye actually penetrates the entire gel, however it only binds permanently to the proteins. • Excess dye is washed out by 'destaining' with acetic acid/methanol, also with agitation. Properly stained/destained gels should display a pattern of blue protein bands against a clear background. The gels can be dried down or photographed for later analysis and documentation. 37 Silver Staining → Silver Nitrate • Silver staining is generally used when detection of very faint proteins is necessary. • Very sensitive. Silver staining methods are about 10-100 times more sensitive than various Coomassie Blue staining techniques. • The most commonly used source of Ag+ is AgNO3. However, the use of silver nitrate usually gives high background but the use of various types of sensitizers such as thiosulfate ions has been shown to decrease the background and increase detection sensitivity. • After fixing the proteins by methanol/acetic acid the sensitizer is added to the gel which chelates the silver. Silver ions binds to the proteins and can easily and specifically be reduced to solid silver (Ag) by a reducing agent such as formaldehyde under alkaline conditions (e.g. Na2CO3). This reaction will stain the protein bands giving them a brown color. The reaction is stopped by adding acid. 38 Step Reagent Volume Time 1 Fixing 40%(v/v) Ethanol / 10% Acetic Acid (v/v) (80 ml EtOH/20 ml HAc) (place gel onto the solution in a glas tray, gel swims surface side down) 200 ml 30 min 2 Incubation 30% Ethanol; 0.2% Sodium Thiosulphate;0.5 mol/l sodium acetate; 0.125 % Glutardialdehyd (w/v) (60 ml EtOH/0.4 g Thiosulfate/13.6 g NaAc/1 ml Glutardial.) 200 ml 30 min 3-5 Washing H2O dist (place gel on the glas tray bottom surface side up) 3 x 200 ml 3x5 min 6 Silvering 0.25% AgNO3 /0.015% Formaldehyde (w/v) (0.5 g AgNO3/80 µl Formaldehyde) 200 ml 20 min 7,8 Washing H2O dist 2 x 200 ml 2x1 min 10 Developing 2.5 % Na2CO3 / 0.015% Formaldehyde (5 g NaCO3/80 µl Formaldehyde) 2 x 200 ml 3-5 min 11 Stopping Preserving 10% HAc, 10% Glycerol (20 ml HAc/20 ml Glycerol) 200 ml 20 min Drying air dry , on the support-film then roll on a polyester-sheet --- 16 h 39 40 Analysis of bands 41 Applications • Estimated molecular masses and relative abundance of unknown polypeptides in a complex mixture • Patterns of bands that suggest presence of isoenzymes or specific complex proteins • Effectiveness of a separation procedure during cell/tissue fractionation • Effectiveness of a procedure to purify specific organelles, proteins, or polypeptides • Condition of a preparation such as extent to which proteins have degraded • Similarity of one preparation to another • Changes in gene expression during developmental stages or resulting from experimental intervention 42 Troubleshooting 43 Critical Parameters and Troubleshooting • It is important to use only high-quality electrophoresis-grade reagents when running the gels. Acrylamide and bisacrylamide both break down in solution to acrylic acid, which affects the mobility of molecules through the gel matrix. • Acrylamide solutions should be protected from light and should not be stored for more than a few months. • Acrylamide monomer is a potent neurotoxin. Do not mouth pipette acrylamide solutions, and wear gloves when handling unpolymerised solutions • Ammonium persulfate should be made fresh. • Clean plates are also essential in order to avoid the introduction of bubbles into the gel when pouring. 44 Smiling Bands The lanes in the center of an overheated gel run faster than the lanes at the sides. This is caused by uneven dissipation of heat by the gel. There are several ways to avoid smiling, the simplest of which is to run the gel at lower voltage. An alternative is to use an apparatus that incorporates a mechanism such as a metal plate to disperse heat evenly throughout the gel, or an active cooling mechanism 45 High Sample concentration 46 Too much Salt / Buffer in the Sample After the samples were filled in the sample-wells and the voltage is applied sample jump out of its slot and run over the stacking gel. This happens when there are too much ions in the sample volumes. It can also happen that ´salty samples´ leave their slots even without an applied electric field. To overcome this, urea is added. If urea is not the solution then the sample is passed through a special desalting column 47 Smeared Bands Smearing can have a variety of causes, but most commonly it is due to an unevenly poured acrylamide mixture or due to gross overloading of protein. 48 Western Blotting • Introduction • Basic Concepts and Method • Troubleshooting 49 Introduction 50 • The term “blotting” refers to the transfer of biological samples from a gel to a membrane and their subsequent detection on the surface of the membrane. • Western blotting (also called immunoblotting because an antibody is used to specifically detect its antigen) is now a routine technique for protein analysis. • The specificity of the antibody-antigen interaction enables a single protein to be identified in the midst of a complex protein mixture. • Western blotting is commonly used to positively identify a specific protein in a complex mixture and to obtain qualitative and semiquantitative data about that protein. 51 Western blotting evolved from Southern blotting, invented by Edwin Southern at University of Edinburgh in 1975, then Northern blotting, invented by George Stark's Stanford group in 1977. 1079: Neal Burnette’s group (at Fred Hutchinson), Harry Towbin's group (in Switzerland) and George Stark's group (at Stanford) Stark's group published first. Towbin's group developed what appears to be the most common method, including the electrophoretic transfer method and buffers, as well as the use of secondary antibodies. Burnette gave the technique the name among other modifications. 52 Basic Concepts and Method 53 • Proteins are separated by gel electrophoresis, usually SDS-PAGE. • The proteins are transferred to a sheet of special blotting paper called nitrocellulose. Transfer may be done by any of the following methods: 1. Wet Transfer 2. Semi Dry Transfer 3. Dry Transfer 54 Wet Transfer 55 Semi Dry Transfer 56 Dry Transfer 57 58 • The blot is incubated with a generic protein (such as milk proteins) to bind to any remaining sticky places on the nitrocellulose. • An antibody is then added to the solution which is able to bind to its specific protein. 59 Detection 60 • The antibody has an enzyme (e.g. alkaline phosphatase or horseradish peroxidase or a fluorescent probe) or dye attached to it which cannot be seen at this time. • The location of the antibody is revealed by incubating it with a colorless substrate that the attached enzyme converts to a colored product that can be seen and photographed. 61 62 63 Troubleshooting 64 High Background Antibody concentration too high Insufficient blocking Cross reactivity of antibody with the proteins in the blocking buffer Membrane got dry Contamination of buffer Contaminated equipments 65 Weak or no signal Proteins did not transfer properly Insufficient binding to the membrane Insufficient amount of antibody Insufficient amount of antigen Antigen masked by blocking buffer Presence of azide (in case of HRP detection) Reaction time is too short Substrate / Enzyme has lost activity 66 Non specific binding Antibody concentration too high Polyclonal antibody used as secondary antibody Diffused bands Antibody concentration too high Too much sample loaded 67 • http://www.currentprotocols.com/WileyCD A/CurPro3Video/videoId-694565070.html 68 Further Reading Physical Biochemistry : Applications to Biochemistry and Molecular Biology by David Friefielder 2nd Edition Publisher: New York : W.H. Freeman, ©1982 Gel Electrophoresis of Proteins: A Practical Approach by BD Hames & D Rickwood Publisher: IRL Press Ltd. Oxford, Washington DC ©1981 Introduction to Biotechnology by W.J. Thieman and M.A. Palladino. Pearson & Benjamin Cummings 69