Mycoplasma Detection - The Stem Cell Training Course
advertisement

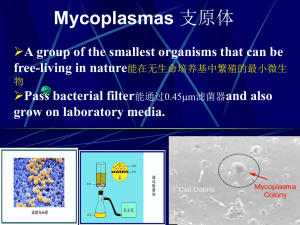
Lonza BioResearch Rapid Mycoplasma Testing June 2012 Content Mycoplasma – An Introduction Mycoplasma Testing for Research Other tools slide 2 What Contaminations Can Be Seen in the Microscope? Bacteria Yeast Fungus Mycoplasma Small black specks Oval/round in shape Filamentous strands Invisible Web-like mesh Only visible via electron microscopy pH change Often mistaken as cell debris Smaller than cells Reflect light (“bright beads”) Formed branched chains Definite movement slide 3 What Are Mycoplasma? Smallest, simplest prokaryotes Lack of rigid cell wall Size ranges from 0.2 to 0.8 µm Many species cannot be removed by filtration Cannot be visualized even at very high concentrations Not affected by traditional antibiotics used in cell culture Limited biosynthetic capabilities Utilize nutrients from “hosts” slide 4 Effects of Mycoplasma on Cells Increased sensitivity to inducers of apoptosis Inhibition of cell growth Alteration of DNA transfection efficiency Chromosomal aberrations Inhibition of cell metabolism Disruption of nucleic acid synthesis Production of viruses compromised Changes in cell membrane antigenicity CELL DEATH DNA fragmentation due to Mycoplasmal nucleases NOT apoptosis slide 5 Mycoplasma Impair Transfection Efficiency HeLa cells infected with Mycoplasma fermantans HeLa cells uninfected Program I-013 no DNA no program + DNA 57.7% GFP+ Program I-013 + DNA 23.0% GFP+ Preliminary data kindly provided by customer slide 6 Mycoplasma – Types & Frequency Acholeplasma laidlawii (bovine): 5-20% Mycoplasma orale (human): 2040% Mycoplasma hyorhinis (swine): 10-40% Mycoplasma arginini (bovine): 20-30% Mycoplasma fermentans (human): 10-20% Mycoplasma hominis (human): 10-20% slide 7 Mycoplasma – Prevalence & Sources Prevalence 15-35 % of continuous cell lines 5% of early passage cell cultures 1% primary cell cultures Sources Cross-contamination from infected cultures Laboratory personnel Culture reagents (e.g. bovine serum) Original isolate tissue (<1%) slide 8 The Speed of An Infection MycoAlert™ MycoAlert® Ratio Ratio 1000 100 10 1 16 h 22 h 40 h 46 h 64 h 70 h 136 h 0.1 Time after innoculation K562 control M. hyorhinis M. salivarium slide 9 Content Mycoplasma – An Introduction Mycoplasma Testing for Research Other tools slide 10 Mycoplasma – Classical Detection Methods Non-luminescence methods Agar culture test (gold standard) PCR methods 2 – 3 weeks Often done externally 4-5 hours Species detection depends on primer set Also detects dead mycoplasma Hoechst stain Time-consuming, poor indicator Experience required slide 11 MycoAlert™ Mycoplasma Detection Kit Unique 20 min bioluminescent assay for mycoplasma detection in cell cultures Mechanism: The assay detects the activity of two enzymes found in mycoplasma and other mollicutes Enzymes are associated with energy generation pathways that result in ATP synthesis The enzymes are found in all 6 of the main mycoplasma cell culture contaminants and the majority of mollicute species Being an enzyme assay, the MycoAlert™ Kit only detects viable mollicutes The enzymes are not found in eukaryotic cells slide 12 The MycoAlert™ Reaction Specific mollicute substrate Photinus pyralis (The Fire Fly) Mycoplasma enzymes ATP Luciferase + Luciferin + O2 LIGHT + Oxyluciferin + AMP + PPi + CO2 Luciferase reaction: Very sensitive (detection of 50 attomols ATP) Wide dynamic range (six orders of magnitude) Amount of light is proportional to the ATP present Compatible with 96-well and 384-well formats slide 13 MycoAlert™ Protocol MycoAlert™ Substrate Specific Substrate for Mollicutes 10 min MycoAlert™ Reagent Lysis, Luciferase, Luciferin Sample 100 µl 5 min Read B A Read B/A < 0.9 = negative B/A > 1.2 = positive 0.9 < B/A < 1.2 = quarantine slide 14 MycoAlert™ Kit – Assay Kinetics MycoAlert™ Reagent added Reading A Reading B + MycoAlert™ Substrate added Clean sample 10000 Infected sample* RLUs 1000 100 10 1 0 2 4 6 8 10 12 14 16 Time (minutes) *Infected sample = supernatant from K562 cell culture infected for 72 hours with M. hyorhinis slide 15 MycoAlert™ Kit – Species Testing Species Result Species Result Species Result Acholeplasma laidlawii positive Mycoplasma canadense positive Mycoplasma lipophilum positive Acholeplasma modicum positive Mycoplasma cloacale positive Mycoplasma muris positive Acholeplasma morum positive Mycoplasma conjunctivae positive Mycoplasma neurolyticum positive Mesoplasma entomophilum positive Mycoplasma equirhinis positive Mycoplasma opalescens positive Mesoplasma florum positive Mycoplasma faucium positive Mycoplasma orale positive Mycoplasma alkalescens positive Mycoplasma fermentans positive Mycoplasma pirum positive Mycoplasma arginini positive Mycoplasma gallinacium positive Mycoplasma pneumoniae positive Mycoplasma arthritidis positive Mycoplasma gallisepticum positive Mycoplasma primatum positive Mycoplasma bovirhinis positive Mycoplasma genitalium positive Mycoplasma pulmonis positive Mycoplasma bovis positive Mycoplasma hominis positive Mycoplasma salivarium positive Mycoplasma bovoculi positive Mycoplasma hyopneumoniae positive Mycoplasma spermatophilum positive Mycoplasma buccale positive Mycoplasma hyorhinis positive Mycoplasma synoviae positive Mycoplasma californicum positive Mycoplasma hyosynoviae positive Spiroplasma citri positive Mollicute species obtained from the National Collection of Type Cultures UK 39 of 118 species in the collection tested to date slide 16 MycoAlert™ Results MycoAlert™ Assay compared to a PCR kit 100 Ratio 10 1 0.1 K562* U937* HL60 JURKAT CHO* BJAB COS7* MycoAlert™ 39 32 0.4 0.4 23 0.8 25 PCR + + - - + - + * Positive cell lines (infected with M. hyorhinis) slide 17 MycoAlert™ Kit – No Interference with Media Components Influence of different media components on MycoAlert® Results: ove 's Isc EM EM py ruv ate So diu m am ate Glu t ess en t ia la min oa cid s ser um No n 20 % Try ps in /ED TA O DM S 10 % Pe n/S tre p I 1 RP M Ratio 10 0,1 Mycoplasma negative Mycoplasma positive (addition of M. faucium) slide 18 Instrumentation Cuvette/tube luminometers Single sample throughput E.g. Lucetta™ Luminometer with tailor-made MycoAlert™ Mode Plate-reading luminometers Up to 96 samples per plate Can be semi-automated if fitted with reagent injectors for high sample throughput But some are not sensitive enough for MycoAlert® measurements Scintillation counters can be used in luminescence mode List of MycoAlert® compatible luminometers is available at: www.lonza.com/mycoalert slide 19 Content Mycoplasma – An Introduction Mycoplasma Testing for Research Other Tools slide 20 Summary – Lonza Products Lonza offers a complete product portfolio for managing mycoplasma contaminations: Detection: Elimination: MycoAlert™ Kit and MycoAlert™ Plus Kit for basic research Lucetta™ Luminometer MycoTOOL® PCR Kit for final industrial release testing MycoZap™ Elimination Kit Prevention: Specific MycoZap™ Antibiotics MycoZap™ Spray (EU only) slide 21 Support Tools Please contact the Scientific Support Team for additional assistance or to request a MycoAlert™ Demo scientific.support@lonza.com scientific.support.EU@lonza.com Other helpful tools www.lonza.com/mycoplasma www.lonza.com/mycoalert (for luminometer list) www.lonzabio.com/faq www.lonza.com/webinar-archive www.lonza.com/mycotool www.lonza.com/mycotestserv slide 22 Thank you for your attention