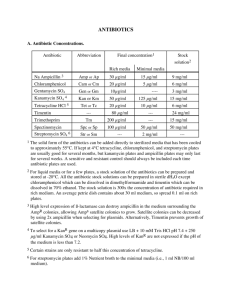
Aminoglycosides
advertisement

Aminoglycosides Intro Group of antibiotics used in the treatment of bacteria infections aerobic G-ve Consists of 2 or more amino sugars and a hexose nucleus Serious toxicity is a limiting factor for their application Streptomycin was the first to be discovered in 1943 by Schatz, Bugie and Waksman Other examples are: Gentamicin* Streptomycin Amikacin Neomycin Netilmicin* Tobramycin Kanamycin Paromomycin+ *Not from Streptomyce spp (from Actinomycetes spp) + Antiparasitic ( amoebiasis, cryptosporidiosis) Families: Determined by the type of amino sugar Neomycin – there are 3 amino sugars attached to 2-deoxystreptamine e.g Neo B, Paromomycin Kanamycin family – 2 amino sugars attached to 2 deoxystreptamine. E.gs amikacin*. Kanamycin A & B, tobramycin *a semisynthetic derivative of kanamycin A and netilmicin is also semisynthetic Aminoglycosides family Gentamicin family– – – – Gent Ci, Gent C1a and C2, sisomicin and Netilmicin (derivative of sisomicin) Streptomycin family – Streptomycin and – dihydrostreptomycin. – Contains streptidine instead of deoxystreptamine Spectrum of activity Aerobic G-ve bacteria ( Citrobacter, Enterobacter, E. coli, proteus, Pseudomonas, Enterococci and Staph aureus *) Lack activity against most anaerobic or facultative bacteria and activity against G+ve# organisms is limited * in combination # Strept pyogenes is highly resistant Mechanism of Action Bactericidal antibiotics Penetration involves active transport Inhibition of protein synthesis by binding to the 30S subunit of ribosomes Causes misreading and premature termination of protein synthesis Resistance May be plasmid mediated inactivation by microbial enzymes or failure of drug penetration Synthesis of metabolizing enzymes Mutation may alter ribosomal binding site for the aminoglycosides Cross resistance with other aminoglycosides may occur Absorption, Distribution and Elimination Polar agents with poor oral absorption Usual routes: IM or I.V Cmax achieved within 30-90 of IM Absorption increases in inflammation No significant amount in breast milk Plasma protein binding is minimal Vd approximates 25% of lean body weight Abs, Distr and Elimination Penetration of CNS: 10-25% of plasma level Accumulates in the perilymph and endolymph as well as renal cortex Vd increases in – leukaemia Clearance increases and T1/2 reduces in cystic fibrosis T1/2 for most; 2-3 hours Elimination is by glomerular filtration Both haemo- and peritoneal dialysis remove aminoglycosides Unwanted effects Ototoxicity: netilmicin is reputed to be mildest on both Vest and Audi. Functions* Nephrotoxicity# Other neurotoxic effects – optic neuritis, peripheral neuritis, neuromuscular blockade Others: angioedema, skin rash, blood dyscrasia, eosinophilia, fever, stomatitis, anaphylaxis *Neo/Amk/kan affect Audi more than others while Str/Gen tend to affect Vest fn more # Gen/Tob/Neo are relatively more nephrotoxic than the others NB: Nephrotoxic effects occurs in 5-10% of patients Therapeutic drug monitoring Necessary in: Patients with life threatening infections Renal impairment 24 hours into new regimen Neonates Samples usually taken just before and 30 minutes after a dose Caution in: Pregnancy Myasthenia gravis (MG) Renal impairment Parkinson’s dx 8th cranial nerve disease Streptomycin Usual dosage: 15-25 mg per Kg body wt IM Therapeutic applications in: Bacterial endocarditis from enterococcal and group D Strep Tularemia Plague Tuberculosis Gentamicin Inexpensive and reliable efficacy Usual dose; 3-5 mg per Kg body wt in 3 divided doses daily Therapeutic Applications: UTI, Pneumonia (nosocomial), Peritonitis, meningitis and sepsis Tetracyclines Tetracyclines Broad spectrum antibiotics (incl: Legionella spp, Ureaplasma, Mycoplasma, chlamydia plasmodium and rickettsial infections) Origin: Streptomyces spp Examples: Chlortetracycline, demeclocyline, oxytetracycline, doxycline*, tetracycline*, minocycline* * semisynthetic Mechanism of action: Binding of the 30S subunit of ribosome, preventing the access of aminoacyl tRNA to the acceptor site on the mRNA-ribosome complex Resistance Plasmid mediated decrease accumulation of the drug Blockade of access by ribosome protecting protein Enzymatic inactivation of TCN ABS, DISTR and ELIMINATION Most are incompletely absorbed when taken orally* Abs occurs mainly in the stomach and upper small intestine Fasting improves abs while presence of food or divalent cations reduce Peak conc ~ 2-4 hr T1/2: 6-12 hrs+ Widely distributed (incl: RE cells in spleen, liver and bone marrow; also synovial and sinuses bone and dentine and prostate) *Chlortetracycline is worst; minocycline and doxy are best + half life of mino and doxy very long 16-18 hr Undergoes entero-hepatic cycling Most tetracyclines are excreted in urine (doxicycline, an exception) Clinical uses Wide range of bacteria diseases+ – Ricketsial infections – Mycoplasma – Chlamydia + Use often precluded by resistance Unwanted effects GI upset including abd pain, nausea, vomiting diarrhea Photosensitivity Hepatotoxicity Renal toxicity Teeth and bone discolouration Skin rashes Pseudomembraneous colitis Thrombophlebitis (IV) Pseudo-tumour cerebri Leukopenia, Thrombocytopenic purpura Chloramphenicol Chloramphenicol Broad spectrum antibiotic (MIC for sensitive strains < 8 ug/ml) Antimicrobial spectrum: Rickettsial, salmonella infections Mechanism Inhibition of protein synthesis via 50S subunit of ribosome** Resistance Plasmid mediated elaboration of inactivating enzymes (acetyl transferase) ** Other 50S: erythromycin Clindamycin Chloramphenicol Introduced to clinical practice in 1949 Bacteriostatic Fallen out favour in western countries cos it causes aplastic anaemia Main use restricted as eye ointment/drops Poorly dissolves in water requiring that IV is given as succinate ester. The succinate ester is incompletely hydrolysed (70%); hence oral preferred to IV Chloramphenicol Usual oral dose = 50 mg per kg IV usually 75 mg per kg Drug level to be monitored in neonates to < 4 yrs old, elderly, renal impaired patients Recommended peak level 15-25 mg/ml (sample taken 1 hr after dose) Trough level < 15mg/kg (sample taken b4 next dose) ABS DISTR EXCN Well absorbed when given orally, (IM not advised as it is poorly absorbed) Peak conc achieved within 2 hours 60% of plasma found in CSF T1/2 2 hours 10% unchanged in urine, the rest is inactivated by glucuronidation in the liver ADRs Gray baby syndrome (consisting of: VDFlaccidityHypothermia Ashen-gray colour); Gray syndrome Jarisch_Hexheimer reactions when used in brucellosis Bone marrow suppression: – presents with low Hb; – does not predict Aplastic anaemia, – dose dependent (>20g) Risk of leukaemia ADRs Bone marrow aplasia* – Not dose dependent – Unpredictable – commonest with oral (1:24000, least with eye preps (1: ~250000); – may begin weeks after stopping drug Interactions: Phenytoin, phenobarb, Rifampicin, chlorpropamide, dicoumarol *Such effect unknown with Thiamphenicol (a methyl-sulphonyl analogue of Chloramphenicol) The Quinolones Intro Group of broad spectrum antibiotics Also known as DNA gyrase Generally bactericidal May be broadly divided into two groups – Fluoroquinolones – Other quinolones: Nalidixic acid, the oldest member, cinoxacin Mechanism Penetrates bacterial cell easily Inhibition of DNA gyrase – (in eukaroytes is called Topoisomerase II) Prevents DNA replication Blocks transcription Resistance results from: – Increased efflux of drug – Altered DNA gyrase binding site Classes of quinolones 4 generations (plus!) Earlier generations have narrower spectrum 1st generation: Nalidixic acid, cinoxacin, oxolinic acid 2nd generation: ciprofoxacin, enoxacin, ofloxacin, norfloxacin 3rd : sparfloxacin, levofloxacin 4th : gatifloxacin, sitafloxacin ADME General good absorption profile Achieves peak plasma conc. 1-3 hrs Food may reduce rate but not extent of absorption Bioavailability ranges from 50-90% Kidneys involved in excretion Clinical uses UTI Travellers’ diarrhoea Bone, joint soft tissues infections Respiratory infections esp. – Legionella spp – Mycoplasma Mycobacterium spp infections Other organisms: Chlamydia, Brucella ADRs Peripheral neuropathy Tendonitis and tendon rupture can occur Rhabdomyolysis SJS Pseudomembranous colitis Prolongation of QT interval Not recommended in pre-pubertal b’cos of tendency to cause arthropathy The Macrolides The macrolides Many membered lactone ring plus deoxy sugar Bacteriostatic antibiotics Inhibits protein synthesis (50S) Resistance is usually plasmid mediated reduced – Erythromycin – Azithromycin – Clarithromycin macrolides Spectrum of antibacterial activity Mostly Gram +ve Diphtheria Mycoplasma Legionella Mycobacteria Borrelia Macrolides Erythromycin base is susceptible to gastric acid inactivation Thus, it is usually presented in enteric form Poorly penetrates CNS but crosses placenta barrier Plasma protein binding 70-90% Half life is ~ 2 hours Clinical uses include: Toxoplasmosis and cryptosporidiasis in HIV/AIDS – Chlamydia, mycoplasma, pertusis, tetanus, syphilis, H. pylori Erythromycin ADRs Hypersensitivity reactions Cholestatic jaundice* Cardiac arrhythmias Transient hearing loss * Likened to hypersensitivity rxn. Starts ~10 days; GI disturbance; + fever; leukocytosis; eosinophilia; elevated liver enzymes Interactions include inhibition of metabolism of: Digoxin, astemizole, carbamazepine, warfarin