pGLO™ GFP Purification — Electrophoresis and - Bio-Rad
advertisement

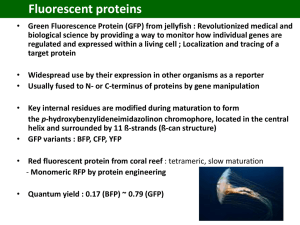
What is next after pGLO™ bacterial transformation? Instructors Stan Hitomi Coordinator – Math & Science Principal – Alamo School San Ramon Valley Unified School District Danville, CA Kirk Brown Lead Instructor, Edward Teller Education Center Science Chair, Tracy High School and Delta College, Tracy, CA Bio-Rad Curriculum and Training Specialists: Sherri Andrews, Ph.D. sherri_andrews@bio-rad.com Essy Levy, M.Sc. essy_levy@bio-rad.com Leigh Brown, M.A. leigh_brown@bio-rad.com Why Teach Bacterial Transformation and Protein Purification? • Powerful teaching tool • Laboratory extensions • Real-world connections • Link to careers and industry • Standards based Workshop Time Line • Introduction • Background on GFP • Protein Electrophoresis of GFP • Purify GFP using column chromatography Starting Point: Transformation Procedure Overview Day 1 Day 2 … Discovery of GFP • Originally Isolated from the jellyfish Aequorea victoria • Naturally occurring in many bioluminescent jelly fish, reef corals and marine crustaceans • Recombinant GFP has 239 amino acids • Expressed as a 26,870 Dalton protein • Barrel structure with the fluorescent chromophore at center of the protein • “Nobel prize-winning” molecule! What is a chromophore? A group of atoms and electrons forming part of an organic molecule that causes it to be colored The GFP chromophore is comprised of three adjacent amino acids. These amino acids are enzymatically converted to an active cyclic chromophore GFP Chromophore Absorbs at 395 nm Emits at 509 nm •In vivo, GFP complexes with aequorin, a calciumbinding protein which transfers energy, to excite GFP •In vitro, UV light is used to excite the GFP chromophore, absorbing light at a wavelength of 395 nm, and emitting at a longer wavelength of 509 nm visible fluorescent green light Recombinant GFP GFP has been mutated • Increased fluorescent photostability • Improved hydrophilicity • Increased solubility • Improved fluorescence • Various colors available • Improved use as a reporter protein Using GFP as a biological tracer or reporter protein http://www.conncoll.edu/ccacad/zimmer/GFP-ww/prasher.html With permission from Marc Zimmer Links to Real-world • GFP is a visual marker • Study of biological processes (example: synthesis of proteins) • Localization and regulation of gene expression • Cell movement • Cell fate during development • Formation of different organs • Screenable marker to identify transgenic organisms www.conncoll.edu/ccacad/zimmer/GFP-ww/GFP-1.htm Protein Electrophoresis of GFP Procedures Overview Students will learn: Protein Electrophoresis of GFP • Prepare an SDS-PAGE sample and understand the components of Laemmli buffer • Understand protein structure and mechanisms for protein folding and unfolding and how different conformations can be identified using electrophoresis • Understand how proteins are separated during gel electrophoresis • Understand the use of electrophoresis in the process of transformation to protein expression • Understand chromophores and the basis of protein fluorescence • Construct a standard curve and determine the molecular weight (MW) of an unknown protein Sample Preparation: SDSPolyacrylamide Gel Electrophoresis (SDS-PAGE) “no heat” samples Labeled tubes: 1. White, no heat 2. Green, no heat 3. White, +heat 4. Green, +heat Samples should be a “healthy scoop” of colonies • Label four screw-capped microtubes • Add 300 µl of Sample buffer to the two “no heat’ tubes • Using the inoculation loop, scrape a sample (20-100 colonies) from an LB/amp (white colonies) plate and transfer to the “White, no heat” tube and mix thoroughly • Using the inoculation loop, scrape a sample (20-100 colonies) from an LB/amp/ara (green colonies) plate and transfer to the “Green, no heat” tube and mix thoroughly Sample Preparation SDS-PAGE “heat” samples • Transfer 150 µl of the “White, no heat” mixture to the “White,+heat” tube • Transfer 150 µl of the “Green, no heat” mixture to the “Green,+heat tube” • Heat the “+heat” tubes to 95°C for 5 min in a water bath. Cool to room temperature Heating the samples denatures the proteins No heating intact chromophore Heating denatured chromophore functional protein non-functional protein fluorescent protein non-fluorescent protein There is an important link between the STRUCTURE and FUNCTION of the protein • Tris buffer – provides appropriate pH CH3 CH2 • SDS (sodium dodecyl sulfate) – Solubilizes and denatures proteins – Adds a negative charge to the protein CH2 CH2 CH2 • DTT – (1,4-Dithiothreitol) reduces disulfide bonds, to help unfold proteins and protein complexes CH2 CH2 CH2 CH2 • Glycerol – Increases the density of the samples to help samples sink into wells of the gel CH2 CH2 S O O O • Bromophenol Blue – dye to visualize samples CH2 O What is in the Laemmli sample buffer? - SDS Load and electrophorese samples 30min at 200V in 1XTGS Buffer UV illumination Coomassie Stain How Does SDS-PAGE Work? • Denatures proteins using detergent, DTT, and heat s-s • Separates proteins based on size • Negatively charged proteins move to positive electrode – + • Smaller proteins move faster through the gel Why Use Polyacrylamide Gels to Separate Proteins? • Polyacrylamide gel has a tight matrix • Ideal for protein separation • Smaller pore size than agarose • Proteins much smaller than DNA – Average amino acid = 110 daltons – Average nucleotide pair = 649 daltons – 1 kilobase of DNA = 650 kD – 1 kilobase of DNA encodes 333 amino acids = 36 kD • Size measured in kilodaltons (kD) • Dalton = mass of hydrogen molecule = 1.66 x 10-24 gram • Average amino acid = 110 daltons GFP VisualizationDuring & Post Electrophoresis During Electrophoresis Post Electrophoresis • Fluorescent GFP can be visualized during electrophoresis Fluorescent isoform • Coomassie stained gels allow for visualization of induced GFP proteins Nonfluorescent isoform Prestained bands + UV activated GFP Coomassie stained bands Determining the molecular weights of GFPs in different conformations GFP Chromatography Kit GFP Purification Procedures Overview Day 1 Day 2 Day 3 Why Use Chromatography? • To purify a single recombinant protein of interest from over 4,000 naturally occurring E. coli gene products. Column Chromatography • Chromatography used for protein purification – Size exclusion – Ion exchange – Hydrophobic interaction Hydrophobic Interaction Chromatography: (HIC) Steps 1–3 1. Add bacterial lysate to column matrix in high salt buffer 2. Wash less hydrophobic proteins from column in low salt buffer 3. Elute GFP from column with no salt buffer Step 1: Hydrophobic Interaction Chromatography • Add bacterial lysate to column matrix in high salt buffer – Hydrophobic proteins interact with column – Salt ions interact with the less hydrophobic proteins and H2O Hydrophobic bead Step 2: Hydrophobic Interaction Chromatography • Wash less hydrophobic from column with low salt buffer – Less hydrophobic E. coli proteins fall from column – GFP remains bound to the column O - O S OO Hydrophobic bead Step 3: Hydrophobic Interaction Chromatography • Elute GFP from column by adding a no-salt buffer GFP – Released from column matrix – Flows through the column Hydrophobic bead Laboratory Quick Guide Helpful Hints: Hydrophobic Interaction Chromatography • Add a small piece of paper to collection tube where column seats to insure column flow • Rest pipet tip on side of column to avoid column bed disturbance when adding solutions • Drain until the meniscus is just above the matrix for best separation Column Chromatography vs. SDS-PAGE for protein isolation and analysis Both methods separate proteins from a complex mixture Column Chromatography Used to isolate a protein from a complex mixture of molecules based on its physical and/or chemical properties HIC separates molecules based on hydrophobicity Need to lyse open the cells to run the soluble proteins over the column Very dilute concentration of GFP in the HIC column fractions SDS PAGE An analytical technique used to detect the presence of a protein of interest Separates molecules based on size Can compare denatured and intact proteins to study protein structure High concentration of GFP from whole cell lysate samples (dense colonies) Both techniques, used in concert can help scientists purify and analytically study proteins Transformation is only the beginning… pGLO Transformation … More techniques are necessary to fully understand the structure and nature of a protein. Protein Purification Size and structure determination Results may lead to more experiments! Webinars • Enzyme Kinetics — A Biofuels Case Study • Real-Time PCR — What You Need To Know and Why You Should Teach It! • Proteins — Where DNA Takes on Form and Function • From plants to sequence: a six week college biology lab course • From singleplex to multiplex: making the most out of your realtime experiments explorer.bio-rad.comSupportWebinars