View ePoster - 2015 AGU Fall Meeting
advertisement

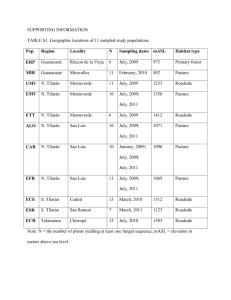
Characterization of active members in C and N cycles in the subsurface environment of the Witwatersrand Basin Poster Number B43G-0499 M. 1 Lindsay , 1Princeton C.Y.M. 1 Lau , G. 1 Tetteh , L. 2 Snyder , T.L. 2 Kieft , B. Sherwood 3 Lollar , L. 3 Li , S. 4 Maphanga , 5 Heerden E. van and T.C. 1 Onstott University - Department of Geosciences, 2New Mexico Tech, Department of Biology, 3University of Toronto - Department of Geology, 4Gold Fields Ltd., 5University of Free State - Department of Biotechnology Abstract Fracture fluid from various depths and locations in Beatrix gold mine (Gold Fields Ltd.), located in the Welkom region on the 2.9 Ga Witwatersrand Basin of South Africa has been previously studied. Research has shown differential geochemistry data and distinctive community structure which varies from the dominance of different Proteobacterial classes in waters with paleometeoric 18O and 2H signatures including methanotrophs to one dominated by Firmicutes including Candidatus Desulforudis audaxviator-like taxa, which are associated with more saline waters with high concentrations of dissolved H2, hydrocarbons from water-rock reaction and 18O and 2H signatures above the Global Meteoric Water Line. Archaea seem to be a minority and all are euryarchaeota including methanogenic genera. The question is:Which of them are actively driving the subsurface C and N cycles? At shaft 3 on level 26, 1.3 kmbls, fracture water from 42 m behind the tunnel wall located in the Main quartzite formation was collected and analyzed. The temperature, pH, Eh, dissolved O2 and salinity of this hydrocarbon-containing fracture water ranged from 35 to 38°C, 8.2 to 8.8, -30 to -100 mV, 0.3 to 30 M and 4.2 to 4.3 ppt, respectively. Gas comprised 60% CH4 and 20% N2. The same fracture formerly yielded Halicephalobus mephisto, the first reported subsurface nematode. Microorganisms were captured on filters in two field seasons. Defined by 16S rDNA, 2011 January sample contains Proteobacteria (50%), Firmicutes (39%) and - and -Proteobacteria (7%). Of the Firmicutes, 90% were represented by Ca. D. audaxviator. All archaea detected are closestly related to sequences also reported from South African gold mines, with Crenarchaeota accounting for 77% of the clones. Prospective methane-oxidation and production were assessed by amplifying genes encoding for particulate methane monooxygenase alpha subunit (pmoA) and methyl-coenzyme M reductase alpha subunit (mcrA). PmoA genes of Type II methanotrophs were found three times more than Type I methanotrophs. A pmoA gene sequence represents 42% of the library matches only and is identical to a putative protein sequence annotated on Ca. D. audaxviator genome, but further analysis is required to validate its candidature of methanotrophy. The cluster of mcrA gene sequences is related to a novel group of anaerobic methanotrophs (ANME) defined by environmental sequences. 2011 July samples from the same borehole revealed an absence of Firmicutes. Two Proteobacterial sequences dominated the bacterial 16S rDNA clone library, accounting for 54% and 25%. The first 16S rRNA clone library for the region confirmed a complete lack of Firmicutes and active Proteobacteria (71% -, 17% - and 6% -Proteobacteria). Only 3% of the active community is confidently inferred as methylotrophs while 22% belongs to N2 fixer Rhizobium sp. which has been demonstrated to stimulate methanotrophic growth and 28% is related to Polymorphum gilvum, which is known for n-alkane degradation. Bacteria Clone Libraries (A) Archaea Clone Libraries BE3 2 6 Ba ct e r ia l 1 6 S r D N A libr a r y ( Ja n u a r y 2 0 1 1 ) (A) BE3 2 6 Ar ch a e a l 1 6 S r D N A libr a r y ( Ju ly 2 0 1 1 ) N=47 N=106 Firmicutes 39% Unidentified archaeon 46% beta-proteobacteria 50% Bacteria 5% Chloroflexi 2% Acidobacteria 1%alpha-Proteobacteria gamma-Proteobacteria 3% Unclassified 4% 1% Uncultured euryarchaeote 19% BE3 2 6 Ba ct e r ia l r D N A libr a r y ( Ju ly 2 0 1 1 ) (B) candidate division OP11 or OD1 1% Active members responsible for CH4 metabolism will be supported by presenting the results of archaeal 16S rRNA, pmoA, mcrA and nitrogenase gene diversities. The lack of Firmicutes in July samples could be attributed to collection methods: different filter membrane, faster flowrate but shorter sampling duration, and less total volume of water filtered. Bacteroidetes 1% Firmicutes 2% Proteobacteria Chloroflexi 1% 2% 3% (B) BE3 2 6 Ar ch a e a l 1 6 S r RN A libr a r y ( Ju ly 2 0 1 1 ) BE3 2 6 Ar cha e a l 1 6 S r RN A libr a r y ( July 2 0 1 1 ) Figure 1. Sampling location. Beatrix Gold mine (28°14'24.06"S, 26°47'45.25"E) is located near Welkom in the Free State province of South Africa, 240 km southwest of Johannesburg on the southern rim of the Witwatersrand Basin. Figure 2. Sample collection at borehole 2 in Beatrix Gold Mine shaft #3, level 26. A sterlized S.S. manifold was attached that allowed the simultaneous collection of different samples at different flow rates. Water Flow Rate (mL/min) 4,000 S ppm 0.66 2.7 Dissolved Trace ppm ppm organics metals DOC 0.34 Mn 0.31 Formate 0.02 Fe 0.0353 59.8 Acetate 0.028 Lactate <0.04 Mo 0.09 Cr <0.04 Propanoate <0.02 Co Gas % He H2 3.16 0.00078 0.0021 O2 9.68 0.0044 N2 42.91 0.0035 CO AlphaProteobacteria 20% GammaProteobacteria 1% N=105 Methanococci 6% Archaea Primer name 0.37 0.624 Ni Cu <d.l. CH4 0.0036 CO2 41% BetaProteobacteria 69% BE3 2 6 p m oA D N A BE3 2 6 Ba ct e r ia l 1 6 S r RN A libr a r y ( Ju ly 2 0 1 1 ) 56.19 betaproteobacteria 17% 0.034 Zn 0.019 NMHC 0.21 1.38 W 0.0454 0.29 As 0.004 11.1 U <d.l. alphaproteobacteria 71% Uncertain 20% Methanococci Methanomicrobia Bacteria Uncultured euryarchaeote Unidentified archaeon N=103 gammaproteobactera 6% uncultured bacteria 6% Figure 6. Archaeal compositions of BE326 borehole water in (A) DNA and (B) RNA samples collected in July 2011. Both communities reveals prevalence Euryarchaeota of Methanobacterium (SA-12) and unidentified/ uncultured archaeon while the DNA shows less Methanobacterium than the cDNA. Frequency of SA specific archaea within the DNA was 3 Methanobacterium, 3 uncultured archaea SAGMA-F, and 1 other uncultured archaeon. For the cDNA, the only South Africa specific result was in the Methanobacterium, all SA-12. South African methanogens (e.g. Methanobacterium sp. SA-12, OTU belonging to SAGMA-F cluster) Expected amplicon size (bp) ~800 ARC-915R GTG CTC CCC CGC CAA TTC CT BAC-S-8Fa AGR GTT YGA TYM TGG CTC AG BAC-S-926R CCG TCA ATT CMT TTR AGT nifH_PolF TGC GAY CCS AAR GCB GAC TC nifH-PolR ATS GCC ATC ATY TCR CCG GA Methanotrophs A189m A682m_a GGN GAY TGG GAC TTY TGG GAA YSC NGA RAA GAA CGM ~500 Methanogens Mlas mcrA-rev GGT GGT GTM GGD TTC ACM CAR TA CGT TCA TBG CGT AGT TVG GRT AGT ~500 ~900 ~340 Figure 4. Bacterial compositions of BE326 borehole water in (A) DNA sample collected in January 2011, (B) DNA and (C) RNA samples collected in July 2011. Beta-Proteobacteria and Firmicutes (90% was Ca. Desulforudis audaxviator) together accounted for 90% of the DNA community in Jan 2011 sample. BetaProteobacteria remained as the most dominant group in the clone library of bacterial 16S rDNA generated from the DNA of Jul 2011 sample, where as the Firmicutes population became one of the minorities. The diversity obtained from the RNA extracted from the same sample affirmed the prevalence of Proteobacteria in the borehole, yet alpha-Proteobacteria were more active than others. DNA Note: Primers were adopted or modified from literature 39 OTUs DNA 9 OTU’s RNA Unid. archaeon pMC2A35 hydrothermal vent archaeon Methanobacterium (SA-12) hot spring archaeon 9 OTU’s RNA Agrobacterium (alpha) Polymorphum gilvum (alpha Thiobacillus sp. (beta) A bacterium from Beatrix Mine A beta-Proteobacterium A bacterium of Rhodospirillaceae (alpha) 12 OTUs Methylocystis sp. 35% Methylomicrobiu m Figure 9. Composition of pmoA gene clone library of DNA from Beatrix Mine, sample collected in July 2011. Prevalence of D. audaxviator and Methyocystis sp. Functional gene BLAST results Ge ne m cr A ge ne 1 m cr A ge ne 2 n ifH ge n e 1 n ifH ge n e 2 Frequ en cy 6 1 6 1 D escr ipti on Un cu ltu red Un cu ltu red Un cu ltu red Un cu ltu red arch ae on clo ne OL-K R 40 _3 48 -35 1m _m cr A _7 8 arch ae on clo ne OL-K R 40 _3 48 -35 1m _m cr A _7 7 ba cte rium clo ne 200 13 -5 nitr oge n-f ixing ba cte riu m iso late 2 Un cu ltu red ba cte rium clo ne AB 0 2 1 C an dida tu s Desu lf oru dis au da xvia to r M P1 04C Table 3. BLAST results from PCR product of selected metabolic genes, mcrA and nifH. There were only 2 different results for the mcrA gene, both from the same set of clones. The nifH gene revealed the presence of D. audaxviator as well as other nitrogen-fixers. Conclusions and Further Work Figure 7. Shared OTU’s (operational taxonomic unit) between archaea cDNA and DNA samples. Using aligned sequences, similarities were calculated using Mothur (Schloss et al. 2011) and 4 OTU’s had sequences from both the DNA and cDNA data sets. 18 OTU’s had only cDNA -orDNA sequences. • Some differences between cDNA and DNA results for both Archaea and Bacteria demonstrated the composition of active community differs from the total DNA community • The active community specifically had Methanobacterium, uncultured archaeon and bacteria, unidentified archaeon and bacteria, uncultured euryarchaeote, beta-proteobacteria, gamma-proteobacteria, and a large number of alpha-proteobacteria (mostly rhizobia) • Shared OTU’s between cDNA and DNA further confirmed that presence of active members • D. audaxviator found in both DNA results and cDNA nifH results - it is active in the subsurface fixing N2. • No sulphate-reducing bacteria (dsrAB gene) was detected, however, after a few attempts of PCR amplifications. • Positive amplification of the pmoA gene from cDNA library was achieved, yet it was problematic to get them transformed and sequenced. Further results are pending. PCR amplification using multiple primer sets (Primer sequences in Table 2) Acknowledgements Ligation and cloning (pGEM-T Easy Kit, Promega Sequencing and data-analysis Figure 2. Methodology of molecular analyses D. audaxviator 33% n ifH ge n e 3 n ifH ge n e 4 15.8 Primer sequence (5’ – 3’) Methylococcus sp. 10% Bacteria 13% Bacteria 13% 0.0068 6.43 ACK GCT CAG TAA CAC GT Nitrogen-fixers Methanomicrobia 41% Methanomicrobia (C) ARC-109F Bacteria Figure 8. (Ward, 2004) Phylogenetic tree for mcrA genes. The blue sequences represent the dominant strains found in BE326 samples, and are part of Novel group 2. They are also related to the ANME (anaerobic methane oxidation) group, which could indicate that the mcrA gene for these organisms is reversed. Unidentified archaeon 40% Unidentified archaeon 40% Table 2. Primer pairs used in this study Target group N=43 Methanococci 6% Table 1. Water chemistry of borehole waterBE326 as of July 2012 Field Dissolved measurement inorganics DN Temp (°C) 38.1 pH 8.55 DIC Measured Eh -227 SO42(mV) Conductivity 7.15 PO43(mS/cm) Chemet O2 4 NO2(ppm) Chemet Soluble Fe 0.3 NO3(ppm) Chemet Total 0 Mg2+ Fe (ppm) Chemet H2S 0.1 Sr2+ (ppm) Chemet H2O2 0.5 Ba2+ (ppm) Chemet PO4 0.0075 Al3+ (ppm) Gas Flow Rate 50 Si4+ (mL/min) Methanomicrobia 30% Figure 3. 2HH2O and 18OH2O for fissure water for numerous sampling sites, including Beatrix (squares) (Ward et al. 2004). The global meteoric waterline is indicated. Water from Beatrix for both January 2011 and July 2011 fall on trend with the GMWL. Figure 5. Venn diagram of OTU (operational taxonomic unit) distribution between bacterial community in DNA and RNA samples collected in July 2011. Using aligned sequences, similarities were calculated using Mothur (Schloss et al. 2011). Seven OTUs were common to both communities, whereas DNA community contained a higher number of unique OTUs than RNA community. I would like to thank the Princeton University EEB department and the Office of the Dean of the College for their support and funding. Additional support was provided by NSF grant #EAR-0948659 to T.C. Onstott. A very special thanks to Dr. Maggie Lau and Dr. TC Onstott for their constant guidance and feedback on this project.