PowerPoint Presentation - Slide 1
advertisement

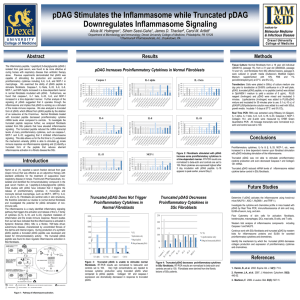
Inflammasome Activation of Dermal Fibroblasts by an Endogenous Peptide Activate Tissue Resident Immune Cells Carol M Artett1, Sihem Sassi-Gaha1, James D Thacker2 1Department of Microbiology and Immunology, Drexel University College of Medicine, Philadelphia, PA 19129 2TherimuneX Pharmaceuticals, Inc., Doylestown, PA 18902 Abstract Results We challenged 42 Swiss Webster mice with a lethal dose of S. typhimurium (5 x103 cfu/mouse) intraperitoneally; and 21 received a μM dose of the natural pDAG (isolated from goat serum) one day prior to the challenge. The mortality of the mice was monitored daily; natural pDAG conferred protection in the treated mice (Fig 2A). Eighty percent of the treated mice survived compared to 10% that did not (P <0.0001 – pooled data from 2 studies). As pDAG induced IL-6 (Fig 3), we measured acute phase protein production in rabbits. We examined the production of antibodies in rabbits challenged with M. tuberculosis (Freund’s Complete Adjuvant, FCA) with or without 15 g of purified pDAG. Six New Zealand White female rabbits received 0.25 ml FCA, however three also received 15 g natural pDAG. Blood was drawn one day prior to the inoculation and on days 3, 5, 7, 10, 12, 14, and 17. Serum was analyzed for total immunoglobulin and M. tuberculosis specific IgM antibodies by ELISA. Natural pDAG induced higher IgM levels than FCA alone (Fig 2B). 100 pDAG+FCA 90 1.4 pDAG IgM Titer, AU Control 60 ATP IL-33 IL-18 K+ efflux acALY18 oligomerization Active NALP3 PYD CARD NF-B pro-IL-33 pro-IL-18 20 0.4 10 0.2 0 1 2 3 4 5 6 7 8 9 0 10 0 Day 3 pro-IL-1 ASC Pro-caspase 1 Inflammasome Proteolytic cleavage p50 p65 Nucleus NF-B p50 p65 NF-B responsive Gene transcription Figure 1. Pathway of inflammasome activation. 10 12 14 17 acALY-18 induced fibroblast secretion of IL-6 and IL-8 IL-1β orchestrates IL-6 and IL-8 secretion (6), and because acALY-18 (synthetic peptide for natural pDAG) induced IL-1β, we measured IL-6 and IL-8 secretion from fibroblasts treated with acALY-18/lipofectamine. We found that after 24 h, IL-6 in the culture media was 230 ng/ml (p < 0.01) and IL-8 was 100 ng/ml media (p < 0.001) significantly more than that which was observed in lipofectamine treated fibroblasts. Conditioned media from fibroblasts treated with acALY-18, induced the expression of inflammatory mediators in THP-1 cells We measured the response of THP-1 cells to acALY-18/lipofectamine after 24 hours. RNA was extracted and inflammasome transcripts; CASP1, IL-18, IL-33, and IL-6 were quantified. All transcripts were then normalized to β-actin (Fig 4A). We found that acALY-18 induced the expression of CASP1 1.2-fold, IL-1β 2.5-fold, and IL-33 1.6-fold (Fig 4A). However, when conditioned media from acALY-18/lipofectamine stimulated cells was used to culture the THP-1 cells, we found that the THP-1 cells differentiated into macrophages and the expression of CASP1 was induced 2-fold, IL-18 3-fold, IL-1β 7220-fold, and IL-33 1338-fold (Fig 4B). It is significant that the expression of these transcripts was found to be exponentially higher with the conditioned media than with THP-1/acALY-18 alone. 250 pDAG does not induce a cytokine storm Protein Media Only pDAG 0.04 ng/ml pDAG 0.4 ng/ml pDAG 4 ng/ml LPS 100 ng/ml IL-1β - - - + +++ IL-2 - - - + + IL-4 - - - - + IL-5 - - - - - IL-6 - - ++ ++++ ++++ IL-7 - - - - - IL-8 - + ++ ++++ ++++ IL-10 - - - - ++ IL-12p70 - - - - + IL-13 - - - - + IL-17 - - - - + GM-CSF - - - + - INF- - - - - +++ TNF-α - - - + +++ G-CSF - - - + ++ MCP-1 - - + ++ ++ MIP-1β - - + ++ ++++ Table 1. Natural pDAG induces pro-inflammatory cytokines from human leukocytes. Buffy coats were isolated from fresh human blood and cultured overnight with natural pDAG or LPS. The cytokine or chemokine profile was determined using the BioPlex Protein Array System (BioRad, Hercules CA). KEY: ++++ 100 fold increase +++ = 25 – 100 fold increase ++ = 5 – 25 fold increase + = 1 – 5 fold increase - = no change from untreated control buffy coat cells). THP1 Relative Expression 200 Relative Expression We have shown that an injection of a nM dose of natural pDAG provided a protective benefit in a lethal bacterial challenge in the mouse. Therefore, we wanted to determine the chemokine/cytokine profile induced by immune cells from a freshly isolated human buffy coat. The buffy coat was incubated overnight in RPMI supplemented with purified natural pDAG. We determined if synthetic acALY-18 or natural pDAG were contaminated by LPS by the following methods: 1. Premixing acALY-18 or natural pDAG with polymyxin B prior to incubating with fibroblasts. We found that IL-6 and IL-8 mRNA continued to be elevated with polymyxin B treated acALY-18 or the natural pDAG isolate. 2. As LPS action requires serum proteins, we incubated acALY18 and natural pDAG in fibroblasts cultures in the absence of FBS. We found that acALY-18 and natural pDAG were still able to induce IL-6 and IL-8 mRNA in fibroblasts in the absence of serum. 3. Boiled LPS is able to maintain its functionality. Therefore we boiled acALY-18 and the natural pDAG product prior to adding them to fibroblasts. IL-8 and IL-6 expression was found to be ablated. These findings confirm that LPS does not contaminate our acALY18 or natural pDAG preparations. Methods Transfection: Cells were plated one day prior to transfection at 80% confluence. Natural pDAG or the synthetic peptide to natural pDAG (acALY-18) was diluted in Opti-MEM I medium to yield a solution of 5 ug/ml. PLUS reagent (Invitrogen, Carlsbad CA) and acALY-18 peptide were mixed and incubated 5 minutes. Lipofectamine (Invitrogen) was added to PLUS-peptide mixture and incubated for 30 minutes prior to use. 10 ul of PLUSpeptide/lipofectamine solution was added to the dish with the culture media. Lipofectamine alone was included as a control. Cells were incubated for 24 h at 37°C, 5% CO2. Conditioned media: Fibroblasts were treated with peptide/ lipofectamine as described, overnight. Culture media was removed and replaced with fresh media and the cells incubated for 3 days. The fibroblast conditioned media was collected and used to culture THP-1 cells for 3 days. Real Time PCR: RNA was extracted using RNeasy kit (Qiagen). IL-1 alpha, IL-1 beta, IL-6, IL-8, IL-18, IL-33, Caspase-1, MCP-1, Collagen 1A1, and β-actin were measured by SYBR Green Quantitative PCR. All message transcripts were normalized to β-actin and control were normalized to 100. Conclusions 10000 300 1000 Caspase-1 IB 7 Fig 2B. Natural pDAG acts like an Adjuvant. All rabbits were treated with FCA, but 3 of the rabbits were given 15 g purified natural pDAG. Rabbits were bled on days, 3, 5, 7, 10, 12, 14, and 17 and total immunoglobulin and IgM specific M. tuberculosis antibodies were measure in triplicate. No difference was observed in total immunoglobulin between the two groups; however, pDAG induced IgM antibodies and by day 3 were significantly greater (p = 0.012). This difference increased with time: at day 5, p = 0.0004 and p < 0.0001 at day 17. Fig 2A. Survival curve of the S. typhimurium challenged mice. 42 Swiss Webster mice were challenged with a lethal dose, 5 x 103 cfu/mouse of S. typhimurium +/- 5 μg natural pDAG, 24 h prior to the lethal inoculation. Mice were maintained and monitored for 10 days. Mortality was observed in the control group on day 4 but not in the natural pDAG group until day 7. By day 10, 80% of the natural pDAG group were alive, compared to 10% in the untreated group; p < 0.0001, pooled data from 2 studies. active Proteolysis Of IB 5 Day IL-1 TLR Inactive NALP3 0.6 30 We therefore investigated the signaling of acALY-18 This data allows us for the first time, to understand the mechanism whereby pDAG is able to stimulate the innate immune system via the inflammasome. IB p50 p65 1 0.8 The inflammasome has recently received attention due to its role in the immune response under cellular stress (3). The fundamental role of the inflammasome is to detect immune danger signals (3). Signals leading to the activation of the inflammasome are poorly understood; however, it is known that the inflammasome is formed by a member of the NLRP (Nacht, LRR and pyrin domain containing protein) family and ASC that activates caspase-1 (CASP1). NLRP3 is the most extensively studied inflammasome as it detects microbial products, cellular stress and endogenous danger signals including extracellular ATP (4), hypotonic stress, or toxins associated with cell injury. The inflammasome modulates the activation of the proinflammatory cytokines, IL-1, IL-18, and IL-33. Danger Signals 1.2 40 Hamm et al (1). reported a serum fraction derived from the goat (Capra hircus) that was effective as an adjunctive therapy with standard antibiotics for treatment of suppurative lower respiratory disease in horses. They reported that 86% of horses treated with the serum fraction, in addition to standard antibiotics, recovered within three weeks, whereas only 10% of horses treated with antibiotic alone recovered (1). As no further analyses was performed on the goat serum, we therefore sought to identify the immunological compound mediating this effect. Indeed, we determined that the compound 1-peptidyl-2,3-diacylglyceride (pDAG) was the efficacious molecule (2). Studies in our laboratory have revealed that pDAG mediates its activity by activating the inflammasome, thereby conferring an advantage to the recipient. Cytosol FCA 80 Introduction Toxins Figure 3. Inflammatory Cytokine & Receptor rtPCR Array mRNA expression from 84 proinflammatory genes. Fibroblasts treated with 3nM acALY-18 showed upregulated (red circles) or down-regulated (green circles) genes > 2.0-fold. Genes upregulated were C8A, CCL2, CXCR4, IL1A, IL1B, IL1RN, and IL-6. Genes down regulated were TLR2 and TLR6. Pooled data from 3 independent experiments. 1.6 70 acALY-18 is not contaminated with LPS As the natural pDAG substrate in the mice challenged with a lethal dose of S. typhimurium and dogs infected with parvovirus was given subcutaneously and we demonstrated that diacylglycerol was a membrane transport moiety (2), we therefore thought it was important to look at the effects of intracellular acALY-18 on fibroblasts as a possible primary effector cell. We treated fibroblasts with 3 nM concentration of acALY-18 plus lipofectamine to aid in the transmembrane transport of the peptide. Cells were harvested after 72 h and assayed using the Inflammatory Cytokine & Receptor rt-PCR Array from SABiosciences (Frederick, MD). This array examines 84 inflammatory genes and the results are presented as a scatter plot (Fig 3). Key genes found to be up-regulated were C8A 6.3 fold; CCL2/MCP1 2.2 fold; CXCR4 3.6 fold; IL1A 4.5 fold, IL1B 8.8 fold; IL1RN 9.3 fold; IL6 6 fold). Two genes were significantly down-regulated and these were TLR2 100 fold and TLR6 18 fold). 1.8 50 Signal acALY-18 upregulated innate immune signaling genes in fibroblasts Natural pDAG Protects Mice from Lethal Bacteremia and acts as an Adjuvant in Rabbits % Survival Activation of innate immunity is an important strategy for host defense as it is an early response to a pathogen derived danger signal. Recently, we discovered a lipopeptide, 1-peptidyl-2,3diacylglyceride, that acts like a danger signaling molecule to induce an innate immune response. The peptide moiety alone (acALY-18) activates the inflammasome inducing the secretion of IL-1β, and subsequently IL-6, IL-8, MIP-1α, and MCP-1 in fibroblasts and keratinocytes. Subcutaneous administration of acALY-18 protected mice from a lethal dose of S. typhimurium (survival: 80% treated vs. 10% untreated, P< 0.0001). We extended our initial observations with acALY-18 to further determine its effect in fibroblasts and subsequent effects on immune cells. Arrays confirmed the upregulation of IL-1β, MIP-1α, MCP-1, and IL-6; C8A, IL1-α, IL-1F8, IL-1R2, IL-1RN were also increased. THP-1 cells responded to acALY-18 fibroblast conditioned media with the up-regulation of CASP1 (2-fold), IL-18 (2.6 fold), IL-33 (7.5 fold), (IL-1β 3600 fold), and IL-6 (2.6 fold). The up-regulation of IL-1β and IL-33 was greater than that observed in acALY-18 treated THP-1 cells (2.5 fold and 1.6 fold, respectively), suggesting that the fibroblast response to acALY-18 can mediate immune cell activation. Additional studies are further investigating the activation of the immune response by acALY-18. acALY-18 could directly translate to a novel therapeutic to enhance non-specific innate immune signaling against pathogens resistant to antibiotics. Results 150 100 THP1 + acALY-18 THP1 + Fib. cond. Media 100 10 THP1 + acALY-18 Fib. Cond. Media 50 1 0 CASP1 IL-18 IL-1beta IL-33 Fig 4A. acALY-18/lipofectamine induced the expression of CASP1, IL-1β, and IL-33 in THP-1 . THP-1 cells were treated with acALY18/lipofectamine for 24 h. RNA was harvested and transcripts measured and normalized to βactin. The response of THP-1 cells to acALY18 was modest. CASP1 IL-18 IL-1beta IL-33 Fig 4B. Conditioned media from acALY18/lipofectamine treated fibroblasts induced the expression of CASP1, IL-1β, and IL-33 in THP-1 cells. THP-1 cells were treated with conditioned media from fibroblasts treated with acALY18/lipofectamine for 72 h or conditioned media from fibroblasts not treated with acALY-18. RNA was harvested and transcripts measured and normalized to βactin. We found that the expression of all these transcripts were exponentially greater than that observed in THP-1 cells treated with acALY-18/lipofectamine (Fig 2A). 1. Natural pDAG confers protection against lethal bacteremia in mice 2. Natural pDAG acts like an adjuvant inducing specific IgM antibodies 3. acALY-18 (synthetic peptide of natural pDAG) induces IL-6 and IL-8 secretion from fibroblasts 4. Natural pDAG does not induce a cytokine storm but upregulates the expression of specific cytokines 5. acALY-18 upregulates the expression of some immune signaling genes in fibroblasts 6. THP-1 cells respond to acALY-18 in a similar manner as fibroblasts, however acALY-18 fibroblast conditioned media exponentially induced the expression of cytokines References 1. 2. 3. 4. 5. 6. Hamm, D., et al. Equine Vet J. 34: 71-5, 2002 Thacker J.D., et al. J Natural Products 725: 1993-9, 2009 Martinon, F., et al. Cell Death Diff 14: 10-72, 2007 Piccini A., et al. PNAS 105: 8067-72, 2008 Ogura Y., et al. Cell 126: 659-62, 2006 Cahill C.M., et al. J Biol Chem 283: 25900-12, 2008