ANTIBODY
advertisement

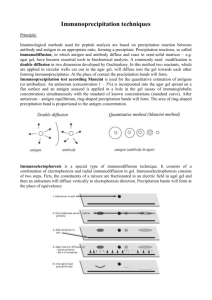
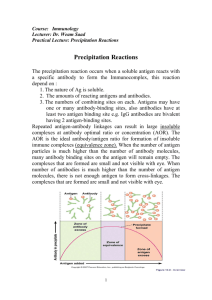
ANTIGEN-ANTIBODY REACTIONS Babitha Elias • Antigens and antibodies combine with each other specifically and in an observable manner. • In the body, they form the basis of antibody mediated immunity in infectious diseases, or hypersensitivity and autoimmune diseases. • In laboratory, they help in diagnosis of infections, in epidemiological surveys, in the identification of infectious agents, enzymes. • Antigen – antibody reactions in vitro are known as serological reactions. Stages of Ag – Ab reactions Primary stage: • Initial interaction between Ag & Ab – invisible • Rapid, occurs at low temperatures & obeys the general laws of physical chemistry & thermodynamics. • Reaction is reversible. • Ag & Ab is bound to each other by weak Van der Waal’s forces, Ionic bonds & Hydrogen bonding. Secondary stage: • Demonstrable events – Precipitation, agglutination, lysis of cells, killing of live antigens, neutralization of toxins, complement fixation, immobilization of motile organisms & enhancement of phagocytosis. Precipitin – Ab participate in precipitation Agglutinin - Ab participate in agglutination Precipitinogen – Ag participate in precipitation Agglutinogen - Ag participate in agglutination Tertiary stage: • Includes neutralization or destruction of injurious agents or tissue damage. • Also includes humoral immunity against infectious diseases as well as clinical allergy & other immunological diseases. GENERAL FEATURES OF Ag – Ab REACTIONS 1. The reaction is specific. 2. Entire molecules react and not the fragments 3. There is no denaturation of the antigen or antibody during the reaction. 4. The combination occurs at the surface. So surface antigens are immunologically relevant. 5. The combination is firm but reversible. The firmness is influenced by the affinity & avidity of the reaction. Affinity – refers to the intensity of attraction between the antigen & antibody molecules. It is the function of closeness of fit between the epitope & antigen binding region of its Ab. Avidity – strength of the bond after the formation Ag-Ab complexes. 6. Both Ags & Abs participate in the formation of agglutinates or precipitates. 7. Antigens & antibodies can combine in varying proportions. Both Ags & Abs are multivalent. MEASUREMENT OF ANTIGEN & ANTIBODY • Measurement may be in terms of mass or more commonly as units or titre. • The Antibody titre of a serum is the highest dilution of the serum which shows an observable reactions with the antigen in a particular test. Two important parameters in serological tests – Sensitivity & Specificity Sensitivity: The ability of the test to detect even very minute quantities of antigen or antibody. • When the test is highly sensitive, false negative results may be absent or minimal. Specificity: The ability of the test to detect reactions between homologous Ags & Abs only, and with no other. • In highly specific test, false positive reactions are absent or minimal. Types of Antigen – Antibody Reactions 1. 2. 3. 4. 5. 6. 7. 8. 9. Precipitation reaction Agglutination reaction Neutralization reaction Complement fixation test Immobilization test Opsonisation Immunofluorescence Radioimmuno assay Enzyme immunoassay PRECIPITATION REACTION PRINCIPLE: When a soluble Ag combines with its Ab in the presence of electrolytes (NaCl) at a suitable temperature & pH, the Ag-Ab complex forms an insoluble precipitate. • When instead of sedimenting, the precipitate remains suspended as floccules – Flocculation reaction • Precipitation can take place in liquid media or in gels such as agar, agarose or polyacrylamide. ZONE PHENOMENON • The amount of precipitate formed is greatly influenced by the relative proportions of Ags & Abs. • If increasing quantities of Ags are added to the same amount of antiserum in different tubes, precipitation is found to occur most rapidly & abundantly in the middle tubes. – Preceding tubes – Ab excess (Prozone) – Middle tubes – Ag & Ab in equivalent proportions (Zone of equivalence) – Later tubes – Ag excess (Post zone) Mechanism of precipitation • Marrack (1934) proposed the lattice hypothesis – mechanism of precipitation • The multivalent antigens combine with bivalent Abs in varying proportions, depending on the Ag – Ab ratio on the reacting mixture. • Precipitation results when a large lattice is formed consisting of alternating Ag & Ab. Marrack’s hypothesis Applications of Precipitation reaction • It can be carried out as either a quantitative or qualitative test. • Sensitive for the detection of Ags. Types of precipitation reactions: – Ring test – Slide test – Tube test – Immunodiffusion – Electroimmunodiffusion RING TEST: • Consists of layering Ag solution over a column of antisera in a narrow tube. Eg: Ascolis thermoprecipitin test, Grouping of Streptococci by Lancefield technique SLIDE TEST: • When a drop of Ag & antiserum is placed on a slide & mixed by shaking, floccules will appear. • Eg: VDRL test & RPR test for syphilis TUBE TEST: • This is employed for the standardization of toxins & toxoids. • Serial dilutions of toxin/toxoid are added to the tubes containing a fixed quantity of antitoxin. • The amount of toxin that flocculates optimally with one unit of the antitoxin – Lf dose. • Eg: Kahn test for syphilis. IMMUNODIFFUSION (precipitation in gel) Advantages of immunodiffusion: • Reaction is visible as a distinct band of precipitation. • Stable, can be stained for preservation. • Indicates identity, cross reactions, non identity between different Ags. 1. Single diffusion in one dimension (Oudin procedure) • Ab is incorporated in agar gel in a test tube & Ag solution is layered over it. • Ag diffuses downward through the agar gel – forming a line of precipitation. 2. Double diffusion in one dimension (Oakley-Fulthorpe procedure) • Ab is incorporated in agar gel • Above which is placed a column of plain agar. • The Ag is layered over it. • The Ag & Ab move towards each other through the intervening column of plain agar & form the precipitate. 3. Single diffusion in two dimensions (Radial immunodiffusion) • Here the antisera is incorporated in a gel & poured on a flat surface. • Wells are cut on the surface to which Ag is added. • It diffuses radially from the well & forms ring shaped bands of precipitation concentrically around the well. 4. Double diffusion in two dimensions (Ouchterlony procedure) • Helps to compare different antisera & antigens directly. • Agar gel is poured on a slide & wells are cut . • Antiserum – central well • Different Ags in the surrounding wells. Reaction of identity Lack of relatedness Partial identity Elek’s gel precipitation 5. Immunoelectrophoresis • Graber & Williams devised this technique. • This involves the electrophoretic separation of composite Ag into its constituent proteins, followed by immunodiffusion against its antiserum – separate precipitin lines. • It is performed on an agarose gel with an Ag well & Ab trough cut on it. • The test serum is placed in the antigen well & electrophoresed for about 1 hour. • Ab against human serum is placed in the trough & diffusion allowed for 18 – 24 hrs. Immunoelectrophoresis ELECTROIMMUNODIFFUSION • • The development of precipitin lines can be speeded up by electrically driving the Ag & Ab. Two types 1. Counterimmunoelectrophoresis (One dimensional double electroimmunodiffusion) 2. Rocket electrophoresis (One dimensional single electroimmunodiffusion) 1. Counterimmunoelectrophoresis (CIE) • • • This involves simultaneous electrophoresis of Ag & Ab in gel in opposite directions resulting in precipitation at a point between them. Produce precipitation lines within 30 mins. Clinical application: detecting Ags like alphafetoprotein in serum, Ags of Cryptococcus & Meningococcus in the CSF. 2. Rocket electrophoresis • Used for quantitative estimation of Ags. • The antiserum to the Ag to be quantitated is incorporated in agarose gel on a slide. • Ag in increasing concentrations, is placed in wells punched in the set gel. • The Ag is electrophoresed into the Ab containing agarose. • The pattern of immunoprecipitation resembles a ROCKET. Rocket electrophoresis Laurell’s two dimensional electrophoresis • Variant of rocket electrophoresis. • The Ag mixture is electrophoretically separated in a direction perpendicular to that of the final rocket stage. • Used to quantitate each of the several Ags in a mixture. THANK YOU