Course: Immunology Lecturer: Dr. Weam Saad Practical Lecture
advertisement

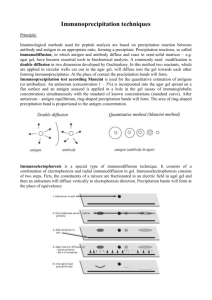
Course: Immunology Lecturer: Dr. Weam Saad Practical Lecture: Precipitation Reactions Precipitation Reactions The precipitation reaction occurs when a soluble antigen reacts with a specific antibody to form the Immunocomplex, this reaction depend on : 1. The nature of Ag is soluble. 2. The amounts of reacting antigens and antibodies. 3. The numbers of combining sites on each. Antigens may have one or many antibody-binding sites, also antibodies have at least two antigen binding site e.g. IgG antibodies are bivalent having 2 antigen-binding sites. Repeated antigen-antibody linkages can result in large insoluble complexes at antibody optimal ratio or concentration (AOR). The AOR is the ideal antibody/antigen ratio for formation of insoluble immune complexes (equivalence zone). When the number of antigen particles is much higher than the number of antibody molecules, many antibody binding sites on the antigen will remain empty. The complexes that are formed are small and not visible with eye. When number of antibodies is much higher than the number of antigen molecules, there is not enough antigen to form cross-linkages. The complexes that are formed are small and not visible with eye. 1 Types of Precipitation Reactions Techniques: Capillary tube precipitation (Ring Test) Ouchterlony Double Diffusion (Immunodiffusion) Radial Immunodiffusion (RID) Immunoelectrophoresis (IEP) Rocket Electroimmunodiffusion (EID) CounterImmunoelectrophoresis (CIEP) 1-Tube precipitation (Ring Test) Qualitative test It is the simplest test among precipitation tests. Clinical example is Milk ring test (MRT), this test is a fast investigation used for cows Brucellosis identification by mixing the cow milk (suspected with Brucella sp. Infection), the blue ring formed after incubation time indicates for Ab present means infection with Brucella sp. present. Principles: 1. Occurs after adding a layer Ag over Ab in a test tube 2. Qualitative: means investigation of the specificity of Ab to the Ag used. 3. Precipitation occurs at the interface between the two reagents (forming a ring). 2 2-Double Immunodiffusion (Ouchterlony Qualitative test) The scientist Ouchterlony (1966) developed this test; the double diffusion technique has many applications e.g. determining whether a given antigen shares structural characteristics (cross-reacts) with other molecules, also; this technique has the advantage that several antigens can be compared around a single well of antibody. Principles: 1. Cutting wells in a gel (agar or agarose); one well contains the antibody and the other well contains the antigens. 2. Antigens and antibodies diffuse towards each other at rates that increase and decrease according to: a) Ag and Ab concentrations in the wells. b) The sizes and molecular weight of both Ag and Ab c) The shape of both Ag and Ab d) The solubility of the Ag. 3. Ag and Ab form a line of precipitation (precipitin line) where they meet at equivalence zoon after incubation time. 4. The gel does not interfere with the diffusion of the Ag and Ab. 5. The formation of the precipitin line in the equilibrium between the antibody and antigen acts as a barrier, which components cannot pass. 6. There are three basic patterns of precipitation as shown in the figure below. The figures show an Agarose gel plates. The antiserum well contains antibodies (blue color) used against the determinants in two different antigens A and B in two different wells (red color), results after diffusion can be one of the followings: A B Ab Figure 1 A B Ab Figure 2 3 A B Ab Figure 3 Figure1: The precipitation lines have fused completely showing the presence of identical determinants between the two Ags. Figure2: Ag A is different from Ag B and both react with the Abs, the precipitin lines cross and a double spur is formed; this is a line of nonidentity determinants. Figure3: Ag A and Ag B share a common determinants but are not exactly the same, a single spur is formed. This is the line of partial identity. 3-Radial Immunodiffusion (RID) (QuantitativeTest) Principles: 1. The technique based on the reaction between an Ag, and a specific Ab. 2. The Ag placed in a well diffuses into a gel ( usually agarose) containing the Ab (e.g. anti-IgG looking for serum IgG). 3. A ring of precipitation around the Ag well will be formed after the Ag-Ab reaction. This ring represents the immunocomplex that formed at equivalence zoon. 4. The gel is prepared by mixing the antibodies with the agarose gel then poured into plate. After the gel become solid, different dilutions of the antigen placed in wells cut into the agarose. 5. A after incubation time, calculating rings diameters, then a standard curve is plotted to determine the concentration of unknown sample Ag. 6. There are two methods of RID: 1. Fahey method 2. Mancini method Mancini method (endpoint): This test is, commonly used in the clinical laboratories for the determination of immunoglobulin levels and complement components in patient samples. The technique of Single Radial Immunodiffusion (SRID) is one of these method techniques. Single Radial Immunodiffusion (SRID) 4 The scientists; Mancini and Oudin developed this technique. This technique used to measure the concentration of Ag in a solution mixed with other Ags when specific antiserum used. The equivalence zoon is called the endpoint because it represents the point where the diameter of the well stops to increase. This endpoint diameter represents the actual concentration of Ag in sample used. Notes: 1. Temperature is a very effective factor in this test. Some reactions need room temperature rather than 37 o C. 2. Single radial immune diffusion need mixing of antiserum with the gel after cooling before pouring in plates. Hence do not need for antiserum well. Experimental design for Double Diffusion Method: In this experiment, human serum will be used as antigen source for albumin protein (Ag) and prepared bovine albumin antiserum (commercial) is used as antibodies source. Procedure: 1. Dissolve agarose gel powder (20 g/l) in boiling phosphate buffered saline PBS, pH 7.4, after cool (50 o C) then pour into special plates, thickness of less than 1mm. 2. After the gel has set, cut out wells about 2mm in diameter. 3. Extract the core by pipette tip with a negative pressure pump. 4. Cover plates with fitted lids, and store in sealed packets at refrigerator (4 o C) until used. 5. Application of serum and commercial prepared bovine albumin antibodies using micropipette 10 µper well for both. 6. Incubation for 24-48 hr. at 37 o C. then read results. 5