solution
advertisement

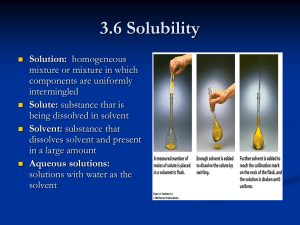
Molecular Biology Technical Skills Skills Micropipetting Preparing solutions Working with concentrations Dilutions Amounts Agarose gel electrophoresis Micropipetting- Measuring small volumes Allows 1 to measure microliters (µL) 000 X less than 1 milliliter 2-20 µL Max. 0.02 mL 50-200 µL 0.2mL 100-1000 µL 1mL Setting the volume- P20 Tens (0, 1=10 or 2=20) Units (0-9) Decimal (1-9 = 0.1-0.9) Setting the volume- P200 Hundreds (0, 1=100 or 2=200) Tens (0, 1-9=10-90) Units (1-9) Setting the volume- P1000 Thousands (0, 1=1000) Hundreds (0, 1-9=100-900) Tens (0, 1-9=10 - 90) Using the micropipettor Step 1 Insert tip Step 2 Press plunger up to first stop Step 3 Insert tip in solution to be drawn Step 4 Draw up sample by slowly releasing plunger Step 5 Withdraw pipettor Dispensing Start dispensing 1st stop =Dispense 2nd stop = Expel Guidelines for optimal reproducibility Use pipettor whose volume is closest to the one desired Consistent SPEED and SMOOTHNESS to press and release the PLUNGER Consistent IMMERSION DEPTH 3-4mm AVOID below surface air bubbles NEVER go beyond the limits of the pipettor Preparing Solutions Definitions Solution Mixture of 2 or more substances in a single phase Solutions are composed of two constituents Solute Part that is being dissolved or diluted – Usually smaller amount Solvent (OR Diluent) Part of solution in which solute is dissolved – Usually greater volume Concentrations Concentration = Quantity of solute Quantity of solution (Not solvent) Three basic ways to express concentrations: Molar concentration (Molarity) Percentages Mass per volume Molarity # of Moles of solute/Liter of solution Mass of solute/MW of solute = Moles of solute Moles of solute/vol. in L of solution = Molarity Percentages Percentage concentrations can be expressed as either: V/V – volume of solute/100 mL of solution W/W – weight of solute/100g of solution W/V – Weight of solute/100mL of solution All represent fractions of 100 Percentages (Cont’d) %V/V Ex. 4.1L solute/55L solution =7.5% Must have same units top and bottom! %W/V Ex. 16g solute/50mL solution =32% Must have units of same order of magnitude top and bottom! % W/W Ex. 1.7g solute/35g solution =4.9% Must have same units top and bottom! Dilutions Reducing a Concentration A Fraction Dilutions Dilution = making weaker solutions from stronger ones Example: Making orange juice from frozen concentrate. You mix one can of frozen orange juice with three (3) cans of water. Dilutions (cont’d) Dilutions are expressed as the volume of the solution being diluted per the total final volume of the dilution In the orange juice example, the dilution would be expressed as 1/4, for one can of O.J. to a TOTAL of four cans of diluted O.J. When saying the dilution, you would say, in the O.J. example: “one in four”. Dilutions (cont’d) Another example: If you dilute 1 ml of serum with 9 ml of saline, the dilution would be written 1/10 or said “one in ten”, because you express the volume of the solution being diluted (1 ml of serum) per the TOTAL final volume of the dilution (10 ml total). Dilutions (cont’d) Another example: One (1) part of concentrated acid is diluted with 100 parts of water. The total solution volume is 101 parts (1 part acid + 100 parts water). The dilution is written as 1/101 or said “one in one hundred and one”. Dilutions (cont’d) Notice that dilutions do NOT have units (cans, ml, or parts) but are expressed as one number to another number Example: 1/10 or “one in ten” Dilutions (cont’d) Dilutions are always expressed with the original substance diluted as one (1). If more than one part of original substance is initially used, it is necessary to convert the original substance part to one (1) when the dilution is expressed. Dilutions (cont’d) Example: Two (2) parts of dye are diluted with eight (8) parts of diluent (the term used for the diluting solution). The total solution volume is 10 parts (2 parts dye + 8 parts diluent). The dilution is initially expressed as 2/10, but the original substance must be expressed as one (1). To get the original volume to one (1), use a ratio and proportion equation, remembering that dilutions are stated in terms of 1 to something: ______2 parts dye = ___1.0___ 10 parts total volume x 2x = 10 x = 5 The dilution is expressed as 1/5. Dilutions (cont’d) The dilution does not always end up in whole numbers. Example: Two parts (2) parts of whole blood are diluted with five (5) parts of saline. The total solution volume is seven (7) parts (2 parts of whole blood + 5 parts saline). The dilution would be 2/7, or, more correctly, 1/3.5. Again, this is calculated by using the ratio and proportion equation, remembering that dilutions are stated in terms of 1 to something: __2 parts blood_____ = ___1.0___ 7 parts total volume x 2x = 7 x = 3.5 The dilution is expressed as 1/3.5 What Does This Mean?? If a solution has a 1/10 dilution the fraction represents 1 part of the sample being diluted added to 9 parts of diluent for a total of 10 parts. If this solution was prepared to a final volume of 110 mL, what volumes of solute and what volume of solvent have to be used? In other words, what is the volume of 1 part and of 9 parts? Dilution Factor EXAMPLE: What is the dilution factor if you add 0.1 mL aliquot of a specimen to 9.9 mL of diluent? The final volume is equal to the aliquot volume PLUS the diluent volume: 0.1 mL + 9.9 mL = 10 mL The dilution factor is equal to the final volume divided by the aliquot volume: 10 mL/0.1 mL = 100X dilution factor Practice Problem What is the dilution factor when 0.2 mL is added to 3.8 mL of diluent? Serial Dilutions If a 1/8 dilution of the stock solution is made followed by a 1/6 dilution what is the final dilution? The final dilution is: 1/8 x 1/6 = 1/48 Dilutions Means to reduce a concentration Dilution: A fraction of the dilution factor Dilution factor = Conc. I have Conc. I want Ex. You have a solution at 25 mg/ml and wish to obtain a solution of 5mg/ml Dilution factor = 25mg/mL = 5X 5mg/mL Dilution = 1/the dilution factor = 1/5 = 1 part/5 parts Total Example How would you prepare 25mL of a 2mM solution from a 0.1M stock? Quantities Quantities are equal to amounts NOT concentrations! Ex 1. Two apples per bag = a concentration Two apples = an amount Ex 2. 10g per 100 mL = a concentration 10g = an amount From concentrations to amounts The concentration indicates the amount in a given volume Ex. 1mM = 1millimole per each liter Therefore the amount in 1 L is 1 millimole What volume of solution would you need to have 0.05 millimoles? Agarose Gel Electrophoresis Separates single stranded or double stranded nucleic acid molecules according to their size and their conformation Separates fragments between 100pb and 10 Kbp Resolving power between fragments ≥100pb Migration on an Agarose Gel Top (-) Well Linear Relaxed Direction of migration Supercoiled Bottom (+) What can be determined from an electrophoresis on an agarose gel? Is there DNA How many conformations How many fragments The size of the fragments Total size of nucleic acid molecule The number of cuts Linear? Circular? Migration Profile on Agarose Resolution Log of the size Resolution 1.0% 1.5% Migration distance Determining sizes 100,000 Fingerprinting Standard Curve: Semi-log Distance (mm) 23,000 11.0 9,400 13.0 6,500 15.0 4,400 18.0 2,300 23.0 2,000 24.0 10,000 Size, base pairs Size (bp) B 1,000 100 0 5 10 15 Distance, mm 20 A 25 30