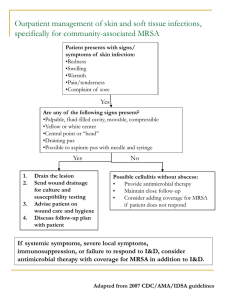
Grumbling problems, etc ,etc
advertisement

Molecular detection of antibiotic resistance Katie L Hopkins PhD katie.hopkins@hpa.org.uk Laboratory of Gastrointestinal Pathogens HPA Microbiology Services Colindale 20th May 2011 Overview Methods used for molecular detection of antibiotic resistance: •In reference labs •Commercially available systems Considerations when choosing a molecular assay: •What are the advantages over phenotypic susceptibility testing? •What are the limitations? Antimicrobial susceptibility testing Antimicrobial susceptibility testing a core function of diagnostic labs. Interpretation of R-patterns can suggest the underlying mechanisms. Limitations: •Time delay due to requirement for pure culture. •May be affected by experimental conditions. •No international consensus on methodology or interpretive criteria. •Low-level resistance can be difficult to detect. Rapid and reliable tests even more important with emergence of MDR organisms. Resistance at the molecular level Genetic basis for antimicrobial resistance includes: 1. Acquisition and expression of new DNA by horizontal transfer. 2. mutations in genes that alter targets or affect gene expression. Informed development of methods: •PCR. •Hybridisation. •Sequencing. Sundsfjord et al . 2004 Services at Colindale Services currently offered by ARMRL include detection of: mecA in S. aureus with borderline methicillin/oxacillin resistance. mupA in mupirocin-resistant S.aureus. 23S rRNA mutations responsible for linezolid resistance in enterococci, staphylococci or streptococci. Genes conferring quinupristin/dalfopristin resistance in enterococci or staphylococci. Genes encoding carbapenemases in Acinetobacter, Enterobacteriaceae* or Pseudomonas spp. (*Send Salmonellae, Shigellae to Laboratory of Gastrointestinal Pathogens). Genes encoding acquired (plasmid-mediated) AmpC β-lactamases in E. coli and Klebsiella spp. resistant to cephalosporins, but with no synergy with clavulanic acid. Services offered by LGP: PCR detection of CLA and TET resistance in H. pylori from culture-negative gastric biopsies. Investigation of the genetic basis of antibiotic resistance in enteric bacteria. •Typically acquired AmpC or ESBL confirmation. “Conventional” PCR Metallo-ß-lactamases Ellington et al. (2007) } Intrinsic to A. baumannii Acquired OXA carbapenemases in Acinetobacter (Woodford et al. 2006; Higgins et al. 2010) Most commonly applied technique. Amplification targets conserved or variable regions within gene of interest. Acquired (plasmid-mediated) AmpCs) (Pérez-Pérez & Hanson, 2002) Separate post-PCR detection – usually agarose gel electrophoresis. PCR + restriction fragment length polymorphism (RFLP) wild-type GCGAGC vs. mutant GCTAGC leads to linezolidR. creates a NheI cutting site in 23S rRNA. Heterozygosity due to multiple copies of 23S rRNA. R S S R R S R R 633-bp 526-bp 430-bp 591-bp 168-bp 96-bp E. faecium / E. faecalis (Woodford et al. 2002) S. aureus (Tsiodras et al. 2001) Real-time PCR (RT-PCR) GIM IMP SIM SPM VIM Metallo-ß-lactamase detection (Mendes et al. 2007) The temperature at which DNA dissociates (melting temperature) is dependent on amplicon length and GC content. Detection of linezolidR E. faecalis/E. faecium (Woodford et al. 2002) Melting temperature is dependent on the degree of complementarity between the probe and target sequence. Commercially available RT-PCR kits Roche Molecular Systems Inc. •LightCycler® MRSA Advanced Test: identify MRSA direct from nasal swabs. •LightCycler® SeptiFast MecA Test: identify MRSA direct from blood samples. •LightCycler® VRE Detection Kit (RUO): identify vanA, vanB, vanB2/3 in VRE (req. DNA extraction). Becton, Dickinson U.K. Ltd./Cepheid SmartCycler® •BD GeneOhm™ VanR: ID of VRE direct from perianal and/or rectal swabs. •BD GeneOhm™ StaphSR: detection and differentiation of MRSA/SA from blood culture, wound and nasal swabs. •BD GeneOhm™ MRSA: direct detection of MRSA from nasal swab. Cepheid GeneXpert system Fully integrated and automated sample preparation, RT-PCR and detection. Specimens don’t need to be batched. <2 mins hands-on time. Results in <1hr – 6 targets per sample. MRSA/SA – orfX-SCCmec junction + mecA + spa. VRE – vanA. MTB/RIF – mutations in rpoB (RUO). http://www.cepheid.com/ DNA probe-based hybridisation assays EVIGENE (AdvanDX): •mecA •mupA •vanA and vanB. No expensive equipment required. No risk of cross-contamination with amplicons. 10 min of hands-on time, with a 3.5-h turnaround time (not incl. DNA extraction). “…the EVIGENE kit was user friendly for the routine microbiology laboratory, with results available within 7 h of recognition of a blood culture positive for GPCC. Rapid and accurate testing of GPCC-positive blood culture samples should facilitate infection control measures, reduce empirical use of vancomycin, and improve the management of MRSA bacteremia…” Levi & Towner, 2003. Strip assays PCR-based reverse hybridisation DNA strip assays (Hain Lifescience). GenoType GenoQuick results within 2.5 hrs. MRSA. results within 4 hrs. MDR + XDR-TB. VRE, MRSA. Helicobacter pylori http://www.hain-lifescience.de/en/ PCR – ELISA: Hyplex assays kits for MßL, MRSA, VRE, ESBLs (TEM, SHV, CTX-M and OXA) and OXA carbapenemases (OXA-23, -40 and -58). identifies genes in 2.5 – 4 hrs directly from clinical specimens. Only one target per well – cost-effective? Avlami et al. 2010 Microarray: Check-Points assays TEM, SHV and CTX-M ESBLs. Plasmidic AmpC. KPC, OXA-48, IMP, VIM, NDM. Can detect SNPs that differentiate between narrow and broad-spectrum ßlactamases. Assay time 6hr. Positive evaluations in: •France (Naas et al. 2011). •USA (Endimiani et al. 2010). •Netherlands (Cohen Stuart et al. 2010). Requires purified DNA. http://www.check-points.com Liquid array: Luminex xTAG assay Detects multiple targets (genes or SNPs) simultaneously. Allele-specific primers adds tag sequence to amplicon – complementary to sequence on bead set. susceptibility in Salmonella Typhi and SPA due to 11 SNPs in gyrA, gyrB and parE (Song et al. 2010). Luminex technology also used in StaphPlex (Qiagen) and MVPlex MRSA (Geneco Biomedical Products). Protocol labour-intensive. Song et al. (2010) Pyrosequencing® technology ‘sequencing by synthesis’ method. Extremely rapid SNP detection – 15min. Built in QC. Can detect novel mutations. Quantifies heterozygotes. Also MTb, FQ-resistance. No commercial assays. Homo-S Hetero-R Homo-R Detecting linezolidR enterococci Sinclair et al. 2003 Phenotypic vs. genotypic: advantages Can be performed direct from clinical specimens: •Rapid. •Good for difficult to culture organisms or slow-growers. •May reduce biohazard risk. Potential for automation. Simple yes/no answer - not dependent on S/I/R categories. Sort out ambiguous phenotypic results. Good for resistance mechanisms that encode low-level resistance. Inform epidemiological studies. Phenotypic vs. genotypic: limitations False –ves due to new mechanisms or mutations. False +ves due to silent genes or partial sequence. Correlation between resistance genotype and phenotype of staphylococci Martineau et al. 2000 Nearly perfect correlation (n = 394): 98% OXA, 100% GEN, 98.5% ERY Low sensitivity when applied directly to clinical specimens. Specificity? Still need culture for confirmation of ID + epidemiological typing. One assay/platform unlikely to cover all resistance mechanisms - cost? Summary Molecular assays for detection of AMR have yielded a wealth of information. Unlikely to replace, but instead augment, phenotypic susceptibility testing. Commercial kits seem to be promising but thorough evaluation in multicentre studies required. Several choices for MRSA, VRE, ESBLs. For now characterisation of new resistance genes and mechanisms are best undertaken in reference laboratories.