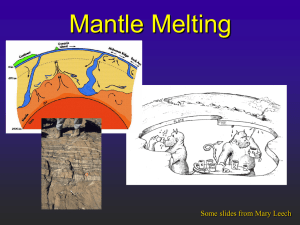
Overheads for mantle composition
advertisement
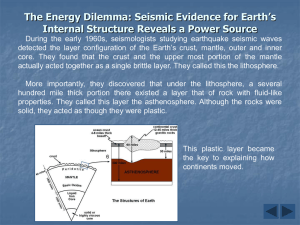
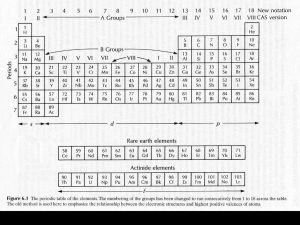
Geological background • 88 elements found in the Earth's crust -- of these, only 8 make up 98%: oxygen, silicon, aluminum, iron, calcium, magnesium, potassium and sodium • In the whole earth, only 4 elements dominate: iron, oxygen, silicon and magnesium • These elements go up to make minerals. A mineral is a naturally occurring, inorganic solid with a characteristic chemical composition and a crystalline structure • Even though there are more than 2500 minerals knows, only nine minerals make up most of the rocks of the Earth's crust - these are the "rock-forming minerals" Normal igneous rock composition: Major element > 1.0 wt. % of the rock or mineral Minor element 0.1 - 1.0 wt. % Trace element <0.1 wt. % (<1,000 ppm) Earth composition continued….. lithophile elements (oxygen, oxides, silicate minerals, Greek lithos - stone) chalcophile (sulphides, Greek khalkos=copper) siderophile (metallic, Greek sideros=iron) The rock-forming minerals • Minerals containing silicon and oxygen are called silicates. These make up more than 95% of the crust. The seven most abundant silicates in the crust are feldspar, quartz, pyroxene, amphibole, mica, clay minerals, and olivine. Olivine and pyroxene are the main constituents of the uppermost mantle. • Most silicates are formed from SiO4 tetrahedra (a silicon atom surrounded by four oxygens) arranged in a variety of ways. Exceptions are quartz and feldspar which are socalled framework silicates. The silicate tetrahedron is exceptionally stable and allows close-packed structures to be formed. The rock-forming minerals (continued) • Olivine is made up of individual tetrahedra, pyroxene and amphibole are made up of chains of tetrahedra, mica and clay minerals are made up of sheets of tetrahedra (giving mica its platey character) • Two other rock-forming minerals are carbonates: calcite (calcium carbonate) and dolomite (calcium-magnesium carbonate) • As low pressure minerals are squeezed, they may suddenly transform to a denser high-pressure phase. Spinel structure (ringwoodite) Undistorted (cubic) perovskite structure Post-perovskite Rocks • Under certain conditions, rocks of the upper mantle and lower crust melt, forming a molten or semi-molten material called magma. Igneous rocks form when this magma cools -- sometimes in surface volcanic eruptions (volcanic rocks) though more often on continents, magma cools and solidifies below the surface forming so-called plutonic rocks • Igneous rock makes up half of the Earth's crust -- the most common igneous rocks are granite and basalt. Rock samples from the mantle are "peridotites" which are dominantly olivine and pyroxene. The Earth’s mantle: How do we know the composition & mineralogy of the mantle? • Cosmochemical constraints • Geophysical constraints • Experimental & theoretical constraints • Direct samples of the mantle - basalts - crystalline samples * alpine/orogenic peridotite * abyssal peridotite * ophiolite * nodules/xenoliths * xenoliths in/& kimberlite/lamproite DIRECT SAMPLES (Peridotite) Common mantle minerals: • Olivine • Orthopyroxene • Clinopyroxene • Al-phase Spinel Garnet Mineralogy of the mantle Lherzolite is probably fertile unaltered mantle Dunite and harzburgite are refractory residuum after basalt has been extracted by partial melting Tholeiitic basalt 15 10 Figure 10-1 Brown and Mussett, A. E. (1993), The Inaccessible Earth: An Integrated View of Its Structure and Composition. Chapman & Hall/Kluwer. 5 Lherzolite Harzburgite Dunite 0 0.0 0.2 Residuum 0.4 Wt.% TiO2 0.6 0.8 Ophiolite emplacement will lead to (pressure-release) melting – this also happens at mid-ocean ridges Figure 1.9 Diagrammatic cross-section through the upper 200-300 km of the Earth showing geothermal gradients reflecting more efficient adiabatic (constant heat content) convection of heat in the mobile asthenosphere (steeper gradient in blue) ) and less efficient conductive heat transfer through the more rigid lithosphere (shallower gradient in red). The boundary layer is a zone across which the transition in rheology and heat transfer mechanism occurs (in green). The thickness of the boundary layer is exaggerated here for clarity: it is probably less than half the thickness of the lithosphere. The Geothermal Gradient Phase diagram for aluminous 4-phase lherzolite: Al-phase = Plagioclase Spinel 50-80 km Garnet shallow (< 50 km) 80-400 km Si ® VI coord. > 400 km Figure 10.2 Phase diagram of aluminous lherzolite with melting interval (gray), sub-solidus reactions, and geothermal gradient. After Wyllie, P. J. (1981). Geol. Rundsch. 70, 128-153. How does the mantle melt?? 1) Lower the pressure – Adiabatic rise of mantle with no conductive heat loss – Decompression partial melting could melt up to 30% Figure 10.4. Melting by (adiabatic) pressure reduction. Melting begins when the adiabat crosses the solidus and traverses the shaded melting interval. Dashed lines represent approximate % melting. • MORB = produced through adiabatic decompression of the upper mantle along the 60,000 km long ocean ridge system Typical Ophiolite Fig. 8-2 (BT) Ophiolite model Have to put the melt back in! • REE (Ringwood)-- pyrolite • Compare major element ratios with meteorites (Jagoutz, Zindler, Hart) -losimag (Can get other elements by using chondritic ratios) Note: All peridotites are metamorphic rocks that have had complex subsolidus history after melt extraction ceased - strain, crystal segregation, deformation, metasomatism, etc. Thus peridotites show compositional variations, particularly in their trace element contents. Nevertheless, they show definite and coherent trends - the least-depleted peridotites (lowest MgO, but highest CaO, Al2O3 and other incompatible trace elements that partition into the liquid phase during partial melting (i.e., fertile) plot closest to the composition of the primitive mantle (PM). Trace element content of the PM has also been estimated basically following similar assumptions and arguments used for the majors. HSE (Os, Ir, Pt, Ru, Rh, Pd, Re, Au) are low in the Earth’s mantle, but not low enough as expected - hence the “late veneer” hypothesis.. Mantle samples Composition of the mantle of the Earth assuming average solar system element ratios for the whole Earth versus PM mantle compositions Ref. solar model MgO 35.8 Al2O3 3.7 SiO2 51.2 CaO 3.0 FeOt 6.3 Total 100 (1) 36.77 4.49 45.40 3.65 8.10 98.41 (2) 38.1 3.3 45.1 3.1 8.0 97.6 (3) 38.3 4.0 45.1 3.5 7.8 98.7 (4) 36.8 4.1 45.6 3.5 7.5 97.5 (5) 35.5 4.8 46.2 4.4 7.7 98.6 (6) 37.8 4.06 46.0 3.27 (7) 37.8 4.4 45.0 3.5 8.1 98.8 (8) 37.77 4.09 46.12 3.23 7.49 98.7 Mg#, molar Mg/(Mg+Fe); FeOt, all Fe as FeO; (RLE/Mg)N, refractory lithophile elements normalized to Mg- and CI-chondrites. References: (1) Palme & ONeil’04 (2) Ringwood’79 = “pyrolite” model (3) Jagoutz et al.’79 (4) Wa¨nke et al.’84 (5) Palme & Nickel’85 (6) Hart & Zindler’86 (7) McDonough & Sun’95 (8) Alle`gre et al.’95 Element abundance in Earth’s mantle – normalized to CI chondrite Proposed structure for oceanic lithosphere Earth composition continued….. Post-accretional chemical planetary processes • shift from low-P to high-P processes on planets • element segregation - grouping of elements, from cosmochemical to geochemical