presentation_6-5-2014-9-52-15
advertisement
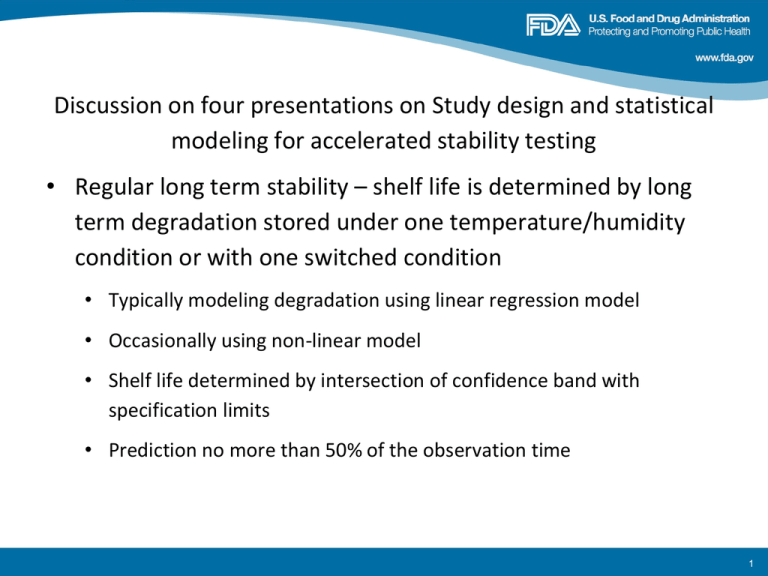
Discussion on four presentations on Study design and statistical modeling for accelerated stability testing • Regular long term stability – shelf life is determined by long term degradation stored under one temperature/humidity condition or with one switched condition • Typically modeling degradation using linear regression model • Occasionally using non-linear model • Shelf life determined by intersection of confidence band with specification limits • Prediction no more than 50% of the observation time 1 • Accelerated stability testing –shelf life prediction based on short storage condition under various combinations of storage conditions such as temperature and humidity. • Provides more information about impact of the storage condition. • Shelf life prediction no more than 10 times of experimental length • Non-linear regression with logistic or Arrhenius model with log transformed data • Determine the confidence band? 2 • FDA statisticians reviewed submissions with accelerated stability testing • Analytical similarity of biosimilar product • Pediatric product development • Post marketing change of a product • To determine the storage strategy in early development stage 3 Some design recommendations for ASAP studies (Tim Kramer) • Methodology for evaluating designs • “Optimum” designs for 3, 4 and 5 environments • Comparison with standard 5-run design • Some extensions • Restricting designs to a subset of parameter space • Errors in humidity and temperature in chambers • Nonlinear degradation • What times and temperatures should be used to optimally determine shelf life (at 25°C/60% RH)? • Although degradation rate parameters are of interest, the main goal is to get a valid estimate of the shelf-life. 4 Design assumptions • Degradation rate fits humidity-corrected Arrhenius equation • Degradation increases linearly with time – Brief consideration of time0.5 or time2 dependency • • • • True shelf life is either 2, 4 or 8 years Activation energy is either 17.2, 25.7 or 34.3 kcal/mol Humidity coefficient (bRH) is 0.00, 0.04 or 0.08 Specification limit is either 0.5%, 1.0% or 2.0% – Pre-exponential factor adjusted to achieve shelf life • Measurement uncertainty is either 6 or 10% of degradation with minimum of 0.02% 5 Activation Energy (kcals/mol) Temperature °C 17.2 25.7 34.3 1.0 1.0 1.0 50 60 4.0 9.5 21.1 8.0 28.7 95.3 16.0 88.2 438.2 70 80 45.0 92.0 295.6 859.4 1983.4 8242.5 25 40 6 Activation Energy (kcals/mol) Temperature °C 17.2 25.7 34.3 25 40 50 60 70 80 1461.0 363.6 154.6 69.2 32.4 15.9 1461.0 182.9 50.9 15.3 4.9 1.7 1461.0 91.2 16.6 3.3 0.7 0.2 7 • Designs to optimally determine shelf life incorporate environments with appreciable degradation – Low temperatures may lead to “no information” results – 4-run designs generally incorporate anchoring of one temperature/humidity combination with 2 points and varying temperature and humidity with other two points 8 John Strong: Experiences and challenges with isoconversional kinetic stability modeling of packaged amorphous solid dispersions Using isoconversional kinetic approach and Moisture-Adjusted Arrhenius Equation to study degradation due to glass transaction temperature of pakaging. The object is to design an experiment such that at each temperature & humidity condition, we know how long it takes to achieve some fractional degradation (i.e. 0.2%), typically the specification limit. Then we can ignore “how” the impurity got to the level of failure for determination of kinetic parameters to be used in further modeling. Raised interesting questions - Constant degradation rate - Small sample parameter estimation - Physical change of dosage form 9 Ken Waterman: A scientific and statistical analysis of accelerated aging for pharmaceuticals: Accuracy and precision of fitting methods • Argue for isoconversion approach for accelerated stability study • Discuss the uncertainty in predictions • Isoconversion methods • Arrhenius • Distributions (MC vs. extrema isoconversion) • Linear vs. non-linear • Conclusions 10 Conclusion: • Modeling drug product shelf-life from accelerated data more accurate using isoconversion • Isoconversion more accurate using points bracketing specification limit than using all points • With isoconversion, regression interval (not confidence interval) includes error of fit, but difficult to calculate with varying SD • Extrema method reasonably approximates RI for interpolation; more conservative for extrapolation • Linear fitting of Arrhenius equation preferred 11 Notes on King, Kung, Fung “Statistical prediction of drug stability based on non-linear parameter estimation” J. Pharm. Sci. 1984;73:657-662 • • • • Used rates based on each time point independently • Changing rate constants not projected accurately for shelf-life • Gives greater precision by treating each point as equivalent, even when far from isoconversion (32 points at 4 T’s gives better error bars than just 4 isoconversion values: more precise, but more likely to be wrong) Non-linear fitting to Arrhenius • Weights higher T more heavily (and where they had most degradation) • Made more sense with constant errors used for loss of potency • Non-linear fitting in general bigger, less symmetric error bars, more likely to be in error if mechanism shift with T Used mean and SD for linear fitting, even when not normally distributed (i.e., not statistically valid method) Do not recommend general use of KKF method (fine for ideal behavior, loss of potency) ken.waterman@freethinktech.com 2014 12 Juan Chen: Comparison of shelf life estimates generated by ASAPPrimeTM with the King, Kung and Fung approach The ASAPprimeTM computerized system is a commercial computer program that analyzes data from accelerated stability studies using a 2-week, productspecific protocol accommodating both temperature and humidity effects through a modified Arrhenius model. The program makes a number of claims: 1. Reliable estimates for temperature and relative humidity effects on degradation rates, 2. Accurate and precise shelf-life estimation, 3. Enable rational control strategies to assure product stability. These claims require careful statistical considerations of the modeling strategies proposed by the system. We evaluate these claims in relation to widely accepted statistical considerations. 13 Conclusion Uncertainty measure for shelf life at accelerated conditions is derived from an “error propagation” calculation using either replicate error or a user defined quantity. Statistical rationale for uncertainty limits is not clear. Does not lead to a statistical confidence statement. Monte Carlo simulation of isoconversion times to predict RT shelf life: Underlying distribution of isoconversion times at accelerated conditions is not documented. The precision of ASAP reported Arrhenius model parameter estimates cannot be validated statistically. Model parameters estimates are influenced by choice of specification limits and user specified SD. R2 reported for overall model fitting is unclear and lacks documentation.