October 15 AP Biology - John D. O`Bryant School of Math & Science
advertisement

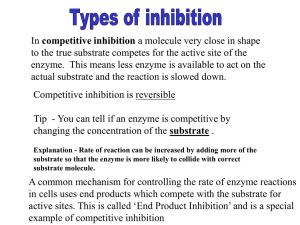
AP Biology John D. O’Bryant School of Mathematics and Science October 15, 2012 AP Biology Agenda Do Now (Quiz) “Why is Patrick Paralyzed?” (case study) Exam 1 discussion “Lorenzo’s Oil” (?) AP Biology Do Now (Quiz) 1. Some bacteria are metabolically active in hot springs because A) they are able to maintain a cooler internal temperature. B) high temperatures make catalysis unnecessary. C) their enzymes have high optimal temperatures. D) their enzymes are completely insensitive to temperature. E) they use molecules other than proteins or RNAs as their main catalysts. AP Biology Do Now (Quiz) 2. Which of the following statements describes enzyme cooperativity? A) A multi-enzyme complex contains all the enzymes of a metabolic pathway. B) A product of a pathway serves as a competitive inhibitor of an early enzyme in the pathway. C) A substrate molecule bound to an active site affects the active site of several subunits. D) Several substrate molecules can be catalyzed by the same enzyme. E) A substrate binds to an active site and inhibits cooperation between enzymes in a pathway. AP Biology Do Now (Quiz) 3. A series of enzymes catalyze the reaction X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme. What is substance X? A) a coenzyme B) an allosteric inhibitor C) a substrate D) an intermediate E) the product AP Biology Do Now (Quiz) 4. A series of enzymes catalyze the reaction X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme. Substance A functions as A) a coenzyme. B) an allosteric inhibitor. C) the substrate. D) an intermediate. E) a competitive inhibitor. AP Biology Do Now (Quiz) 5. The molecule that functions as the reducing agent (electron donor) in a redox or oxidation-reduction reaction A) gains electrons and gains energy. B) loses electrons and loses energy. C) gains electrons and loses energy. D) loses electrons and gains energy. E) neither gains nor loses electrons, but gains or loses energy. AP Biology Do Now (Quiz) 6. Using a series of arrows, draw the branched metabolic reaction pathway described by the following statements. ∙ L can form either M or N. ∙ M can form O. ∙ O can form either P or R. ∙ P can form Q. ∙ R can form S. ∙ O inhibits the reaction of L to form M. ∙ Q inhibits the reaction of O to form P. ∙ S inhibits the reaction of O to form R. AP Biology Do Now (Quiz) 7. According to the figure you created from question 6, which reaction would prevail if both Q and S were present in the cell in high concentrations? A) L → M B) M → O C) L → N D) O → P E) R → S AP Biology Metabolism & Enzymes AP Biology 2007-2008 Factors that Affect Enzymes AP Biology 2007-2008 Factors Affecting Enzyme Function Enzyme concentration Substrate concentration Temperature pH Salinity Activators Inhibitors AP Biology catalase Enzymes and temperature Different enzymes function in different organisms in different environments reaction rate human enzyme hot spring bacteria enzyme 37°C AP Biology temperature 70°C (158°F) How do ectotherms do it? AP Biology pH What’s happening here?! trypsin reaction rate pepsin pepsin trypsin 0 AP Biology 1 2 3 4 5 6 pH 7 8 9 10 11 12 13 14 Factors affecting enzyme function pH changes in pH adds or remove H+ disrupts bonds, disrupts 3D shape disrupts attractions between charged amino acids affect 2° & 3° structure denatures protein (end 10/11) optimal pH? most human enzymes = pH 6-8 depends on localized conditions pepsin (stomach) = pH 2-3 trypsin (small intestines) = pH 8 AP Biology 0 1 2 3 4 5 6 7 8 9 10 11 Salinity reaction rate What’s happening here?! salt concentration AP Biology Factors affecting enzyme function Salt concentration changes in salinity adds or removes cations (+) & anions (–) disrupts bonds, disrupts 3D shape disrupts attractions between charged amino acids affect 2° & 3° structure denatures protein enzymes intolerant of extreme salinity Dead Sea is called dead for a reason! AP Biology Compounds which help enzymes Fe in Activators hemoglobin cofactors non-protein, small inorganic compounds & ions Mg, K, Ca, Zn, Fe, Cu bound within enzyme molecule coenzymes non-protein, organic molecules bind temporarily or permanently to enzyme near active site AP Biology many vitamins NAD (niacin; B3) FAD (riboflavin; B2) Coenzyme A Mg in chlorophyll Compounds which regulate enzymes Inhibitors molecules that reduce enzyme activity competitive inhibition noncompetitive inhibition irreversible inhibition feedback inhibition AP Biology Competitive Inhibitor Inhibitor & substrate “compete” for active site penicillin blocks enzyme bacteria use to build cell walls disulfiram (Antabuse) treats chronic alcoholism blocks enzyme that breaks down alcohol severe hangover & vomiting 5-10 minutes after drinking Overcome by increasing substrate concentration AP Biology saturate solution with substrate so it out-competes inhibitor for active site on enzyme Non-Competitive Inhibitor Inhibitor binds to site other than active site allosteric inhibitor binds to allosteric site causes enzyme to change shape conformational change active site is no longer functional binding site keeps enzyme inactive some anti-cancer drugs inhibit enzymes involved in DNA synthesis stop DNA production stop division of more cancer cells cyanide poisoning irreversible inhibitor of Cytochrome C, an enzyme in cellular respiration stops production of ATP AP Biology Irreversible inhibition Inhibitor permanently binds to enzyme competitor permanently binds to active site allosteric permanently binds to allosteric site permanently changes shape of enzyme nerve gas, sarin, many insecticides (malathion, parathion…) cholinesterase inhibitors AP Biology doesn’t breakdown the neurotransmitter, acetylcholine Allosteric regulation Conformational changes by regulatory molecules inhibitors keeps enzyme in inactive form activators keeps enzyme in active form AP Biology Conformational changes Allosteric regulation Metabolic pathways ABCDEFG 5 6 enzyme enzyme enzyme enzyme enzyme enzyme enzyme 1 2 3 4 Chemical reactions of life are organized in pathways AP Biology divide chemical reaction into many small steps artifact of evolution efficiency intermediate branching points control = regulation Efficiency Organized groups of enzymes enzymes are embedded in membrane and arranged sequentially Link endergonic & exergonic reactions Whoa! All that going on in those little mitochondria! AP Biology Feedback Inhibition Regulation & coordination of production product is used by next step in pathway final product is inhibitor of earlier step allosteric inhibitor of earlier enzyme feedback inhibition no unnecessary accumulation of product ABCDEFG 1 2 3 4 5 6 X enzyme enzyme enzyme enzyme enzyme enzyme AP Biology allosteric inhibitor of enzyme 1 threonine Feedback inhibition Example synthesis of amino acid, isoleucine from amino acid, threonine isoleucine becomes the allosteric inhibitor of the first step in the pathway as product accumulates it collides with enzyme more often than substrate does AP Biology isoleucin e Don’t be inhibited! Ask Questions! AP Biology 2007-2008 Cooperativity Substrate acts as an activator substrate causes conformational change in enzyme induced fit favors binding of substrate at 2nd site makes enzyme more active & effective hemoglobin Hemoglobin 4 polypeptide chains can bind 4 O2; 1st O2 binds now easier for other O2 to bind AP3Biology Lorenzo’s Oil AP Biology