Characteristic-Physical-and-Chemical
advertisement

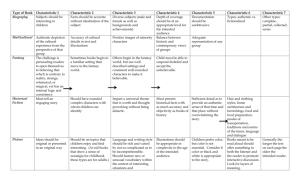
Characteristic Properties of Matter How Are You Identified? Eye Color Hair Color* Skin Color Fingerprints DNA These are all characteristics you have that will stay with you for life and can be used to identify you. *may change due to age or vanity First, A Quick Review Matter is anything that has mass and takes up space. Matter is the “stuff” (atoms and molecules) that makes up the universe Matter is stuff Examples of Stuff (aka matter) Plants, animals, rocks, water, soda, air, oxygen are all examples of matter Matter can be living or non living Matter can be solid, liquid or gas They are all made of atoms and molecules They all have mass and take up space They are all “stuff”. Yet all of the examples of stuff listed above are different They all have different characteristics or properties that can be used to identify them Characteristics of matter can be either physical properties or chemical properties Characteristic Properties of Matter Characteristic properties help scientists identify a particular substance Characteristic properties are always the same for each kind of matter Characteristic properties do not change even though the shape or amount of matter may change Characteristic properties are like the eye color, hair color, skin color, fingerprints and DNA of matter property= an attribute, quality, or characteristic of something. physical property=A quality of a substance that can be observed or measured. chemical property= A quality of a substance that has to do with the way it reacts with other substances What Are Some Physical Characteristic Properties? Physical properties can be seen or measured without changing the identity of the substance identified or described with your senses Melting point, boiling point, freezing point State of matter at room temperature (solid, liquid or gas) Density Magnetic properties Solubility (will it dissolve in another substance?) Conductivity (will it conduct heat, electricity) Ductility, malleability (can it be drawn into a wire or pounded into a thin sheet) What Are Some Physical Characteristic Properties? What Are Some Chemical Characteristic Properties? Chemical properties may not be observed with your senses. determines how a substance interacts with another substance. determine if the substance can change into a new substance with different properties. Flammability Reactivity (how it behaves in the presence of another substance) Oxidation (another word for rust and tarnish) What Are Some Chemical Characteristic Properties? Can You Identify? Physical Properties: Liquid at room temperature 0 Boiling point is 100 C Colorless 3 Density is 1 g/cm That was easy! Let’s Try Another! Physical Properties 3 Density is 8.96 g/cm Insoluble in H2O Ductile, malleable Chemical Properties Oxidizes Reacts with acids Hint? What do you think the flame is made of? Does gold (Au) oxidize? How do you know? Now You’re On Your Own ??????????????????