File
advertisement
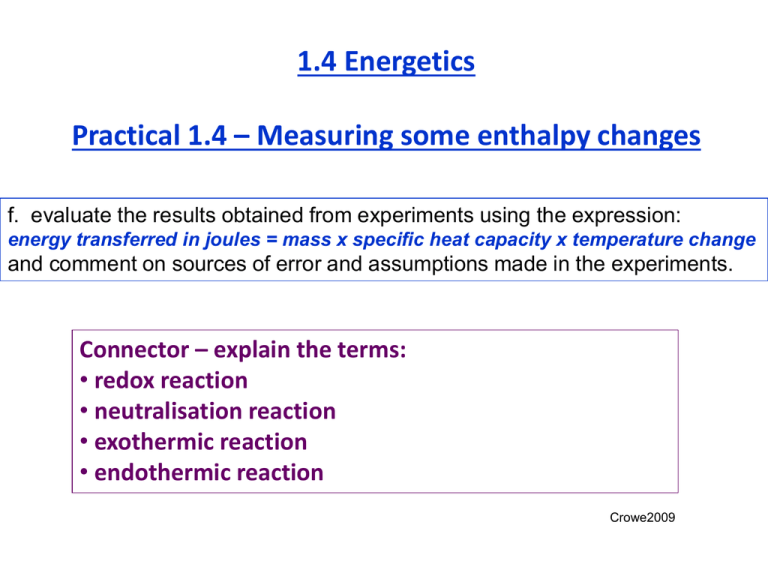
1.4 Energetics Practical 1.4 – Measuring some enthalpy changes f. evaluate the results obtained from experiments using the expression: energy transferred in joules = mass x specific heat capacity x temperature change and comment on sources of error and assumptions made in the experiments. Connector – explain the terms: • redox reaction • neutralisation reaction • exothermic reaction • endothermic reaction Crowe2009 Useful information Usually one reagent is in excess to ensure a complete reaction • So calculations should be based on the fully reacted reagent. Certain assumptions are made during the calculation • The density of the solution and its specific heat capacity is that of water. • That no heat is lost to the surroundings. The main source of error in these experiments is • heat loss to the surroundings – (atmosphere & equipment) Other sources of error include: • incorrect measurements • solution concentrations • mass of reactants Accuracy • Remember to give numerical answers in line with that of the least accurate measurement recorded – don’t just write your calculator’s result with lots of decimal places! • % error = uncertainty in measurement x 100 reading E.g.1 a balance has an uncertainty of 0.01 g when read to the second decimal place. What is the % error for a reading of 2.64g? % error = 0.01 x 100 = 0.38% Note: measurements to 2dp so answer to 2dp 2.64 E.g.2 the temperature rise was 6.1oC, while the thermometer could be read to ± 0.5oC. What was the % error? (Hint: how many readings are required to determine a temperature change?) % error = 2 x 0.5 x 100 = 16.4% 6.1 Note: measurements to 1dp so answer to 1dp Error in measuring temperature changes can be reduced by using graphs produced manually, or by a data logger: A data series is plotted and the max temp change is obtained by extrapolation. The data is plotted in real time and the max temp change is obtained directly. Working out enthalpy changes in solutions Part 1 Calculating the heat absorbed by the solution q= m c x x ΔT E.g. When 50cm3 of 1.0M HNO3 was neutralised by 50cm3 of 1.0M NaOH the following results were obtained: Starting temperature of solns. = 19.0oC Maximum temperature reached = 25.1oC Calculate the heat absorbed by the solution, given that: the mass of 1cm3 of water = 1g; and that the specific heat capacity of water = 4.2 JK-1g-1. q= m x c x ΔT q = 100 x 4.2 x 6.1 = 2562 J Working out enthalpy changes in solutions Part 2 Calculating the enthalpy change 1. Write a balanced equation for the reaction between nitric acid and sodium hydroxide. 2. What are the units for ΔH? 3. What is the definition of the enthalpy of neutralisation? HNO3 (aq) + NaOH (aq) NaNO3 (aq) + H2O (l) kJ mol -1 Enthalpy of neutralisation is the enthalpy change when 1 mole of H+ ions from an acid is neutralised by 1 mole of OH- ions Use the information given in the question and your answer from q = mcΔT to work out the enthalpy of neutralisation for the above reaction. Solution 50cm3 of 1.0M HNO3 was neutralised by 50cm3 of 1.0M NaOH Energy = 2562 J = 2.562 KJ Enthalpy of neutralisation is the enthalpy change when 1 mole of H+ ions from an acid is neutralised by 1 mole of OH- ions No. of moles of H+ ions used = 1.0 x 50 = 0.05 moles 1000 moles Concentration x volume in dm3 Solution 50cm3 of 1.0M HNO3 was neutralised by 50cm3 of 1.0M NaOH Energy = 2562 J = 2.562 KJ Enthalpy of neutralisation is the enthalpy change when 1 mole of H+ ions from an acid is neutralised by 1 mole of OH- ions No. of moles of H+ ions used = 1.0 x 50 = 0.05 moles 1000 So 0.05 moles of H+ ions produced 2.562 KJ energy 1.0 mole of H+ ions will produce 1.0 x 2.562 KJ energy 0.05 ΔH neut = - 51.2 KJ mol-1 Why is the ΔH value –ve? Why is the answer to 1dp? For other questions follow link http://www.studentxpress.ie/educ/chem/chem2/chem2.html