Biochemical Reactions
advertisement

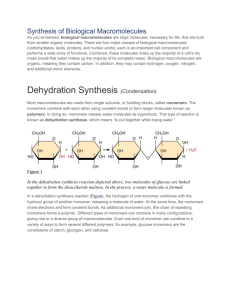
Biochemical Reactions First… a Summary Macromolecules Monomers + functional groups Four types of macromolecules of interest to us: Carbohydrates Proteins Lipids Nucleic Acids Carbohydrates Monomer: simple sugar Ex. Glucose Functional group(s): Carboxyl Hydroxyl Polymer: complex CHO Starch, glycogen Proteins Monomer: amino acids 20 total, 8 or 9 essential Functional group(s): Carboxyl Amino Polymer Polypeptide Protein Lipids Monomer: Fatty acid Functional group(s): Carboxyl Polymers: many – depending on the type of lipid Phospholipid, triglyceride Nucleic Acids Monomer: nucleotide A, T (or U), C, G Functional group(s): Phosphate Amino Hydroxyl Polymer: DNA and RNA Biochemical Reactions Chemical reactions associated with biological processes Often involve a combination of more than one type of reaction Four main types of reactions: Neutralization Oxidation-Reduction Condensation Hydrolysis Acid-Base Reactions Acid: produces H+ ions in water pH Base: produces OH- ions in water or accepts H+ ions pH value less than 7 value more than 7 Neutralization Reaction: interaction of an acid and a base to form a salt (an ionic compound) and water Neutralization Reaction Necessary to maintain a constant pH state within the body Buffers: resist changes in pH H+ ions when fluid is too basic Take up H+ ions when fluid is too acidic Release Oxidation-Reduction Reactions Involves the transfer of electrons Oxidation: loss of electrons Reduction: gain of electrons Electrons are highly reactive and don’t exist on their own in cells If oxidation occurs to one molecule in the cell, reduction must immediately to another molecule The entire reaction is often called a redox reaction Condensation Reactions Involved in the assembly of all four types of macromolecules An H atom is removed from a functional group on one molecule, and an OH group is removed from another molecule Result: a larger molecule + water (water out, monomer in) Also known as dehydration synthesis Hydrolysis Reactions Involved in the breakdown of macromolecules into their monomers Water is added to break the bonds between monomers (water in, monomer out) H from the water is added to one molecule, and the OH group is added to the adjacent monomer Covalent bond between monomers breaks to form two smaller molecules Role of Enzymes An enzyme is a biological catalyst Speeds up a biochemical reaction, but is not used up in the reaction Enzymes are proteins Have a specific shape Each enzyme fits specifically with a substrate (the reactant for the reaction) to form an enzyme-substrate complex Like a lock and key! Enzyme-Catalyzed Reactions Enzymes prepare substrates for reaction by changing the substrate, its environment, or both, in some way Causing bonds to stretch or bend (making them more fragile) Bring two substrates together Transfer electrons to or from the substrate (i.e. reduce or oxidize it), making it less stable Add or remove H+ ions to or from the substrate (i.e. act like an acid or base), destabilizing it Enzyme Denaturation Proteins are called denatured when they have lost their shape. Caused by changes in temperature, pH, environmental factors, etc. Because shape is so important to protein function, denatured proteins are no longer able to carry out their proper function Liver Functions Importance of Catalase H2O2 (hydrogen peroxide) is a harmful byproduct of many of the metabolic processes that take place in the liver Must be removed quickly This is the responsibility of the enzyme catalase Factors Affecting the Action of Catalase What might our liver be exposed to that