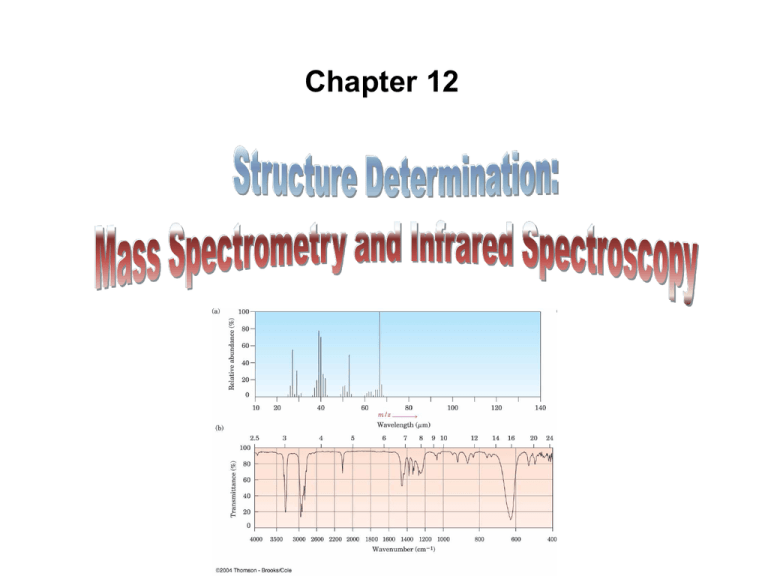
Chapter 12
Introduction
• The analysis of the outcome of a reaction
requires that we know the full structure of the
products as well as the reactants
• Determining the Structure of an Organic
Compound
– In the 19th and early 20th centuries, structures were
determined by synthesis and chemical degradation
that related compounds to each other
– Powerful techniques are now available that greatly
simplify the problem of structure determination
• Physical methods now permit structures to be
determined directly.
• We will examine:
– Mass Spectrometry (MS) — this chapter
– Infrared (IR) Spectroscopy — this chapter
– Nuclear Magnetic Resonance Spectroscopy (NMR) —
Chapter 13
– Ultraviolet Spectroscopy (UV) — Chapter 14
• Mass Spectrometry (MS) – determines the size and formula
• Infrared (IR) Spectroscopy – determines the kinds of
functional groups present
• Nuclear Magnetic Resonance Spectroscopy (NMR) –
– determines the carbonhydrogen framework
• Ultraviolet Spectroscopy (UV) – determines if a conjugated
p electron system is present
1.
Mass Spectrometry
• Mass Spectrometry (MS) – is a technique used to
measure the mass, and thus the molecular weight
(MW), of a molecule
– It also provides structural information about a
molecule from the masses of fragments produced
Mass Spectrometer
• Mass Spectrometers have three basic parts:
– an ionization source
– a mass analyzer
– a detector
Sample
Display
Ionization source
Mass Analyzer
Detector
Sample molecules
are given an
electrical charge
Ions are separated
by their mass-tocharge ratio
Separated ions are
observed and counted
• In the ionization source, sample is vaporized and
bombarded by high-energy electrons that remove an
electron.
• This creates a cation-radical
– Cation: Molecule is positively charged (after losing an electron)
– Radical: Molecule has an odd number of electrons
• Bonds in cation radicals begin to break (fragment).
– Some are positively charged (cations)
– Some are neutral
• The mass analyzer separates the ions by their mass-tocharge ratio (m/z)
– Mass-to-charge ratio (m/z) is measured
• The detector records the fragments as peaks at the various
m/z ratios
– z is usually 1. Thus, m/z is m.
Mass Spectrometer
Mass Spectrum
• Mass spectrum – plots mass of ions (m/z) (x-axis) versus
the intensity of the signal (roughly corresponding to the
number of ions) (y-axis)
• Tallest peak is base peak (100%)
– Other peaks listed as the % of that peak
• Peak that corresponds to the unfragmented radical
cation is parent peak or molecular ion (M+)
MS Examples: Methane and Propane
• Methane produces a parent peak (m/z = 16) and
fragments of 15 and 14 (See Figure 12-2 a)
MS Examples: Methane and Propane
• The MS of propane is more complex (Figure 12-2 b)
since the molecule can break down in several ways
Figure 12-2 a
Figure 12-2 b
2.
Interpreting Mass Spectra
• Mass Spectrometry (MS) – is a technique used to
determine the molecular weight (MW) from the
mass of the molecular ion
• Double-focusing MS instruments - have such highresolution that they provide “exact mass”
– They distinguish specific atoms at an accuracy of 0.0001
atomic mass units
Double-focusing instruments provide “exact mass”
• Example: MW “72” is ambiguous:
– C5H12 and C4H8O have MW = 72 but:
• C5H12 has an exact mass 72.0939 amu and
• C4H8O has an exact mass 72.0575 amu
– This is the result from fractional mass differences of
atoms 16O = 15.99491, 12C = 12.0000, 1H = 1.00783
• Instruments measure the sum of the exact
atomic masses of each isotope in a molecule
• They include computation of formulas for each peak
Other Mass Spectral Features
• Some compounds fragment so easily that no
molecular ion M+ is observed on the mass spectrum
– Example: 2,2-dimethylpropane (C5H12; MW = 72)
No M+ is observed when
electron-impact ionization
is used
Other Mass Spectral Features
• If molecular ion (M+) is not present due to electron
bombardment causing breakdown, “softer” methods
such as chemical ionization are used
• Peaks above the molecular weight (M+1) appear as a
result of naturally occurring heavier isotopes in the
sample (i.e 13C and/or 2H)
(M+1) may be due to:
– 13C that is randomly present (1.10% natural abundance)
– 2H (deuterium) that is randomly present (0.15% natural
abundance)
Practice Problem: Write as many molecular formulas as you can
for compounds that have the following
molecular ions in their mass spectra. Assume
that all the compounds contain C and H and
that O may or may not be present.
a) M+ = 86
b) M+ = 128
c) M+ = 156
Practice Problem: The male sex hormone testosterone contains
C, H, and O and has a mass of 288.2089 amu
as determined by high-resolution mass
spectrometry. What is the molecular formula
of testosterone?
3.
Interpreting Mass Spectral
Fragmentation Patterns
• Fragmentation pattern – is the way molecular ions
break down to produce characteristic fragments
that help in identification
– It serves as a “fingerprint” for comparison with known
materials in analysis (used in forensics) recorded in a
computerized data base called the Registry of Mass
Spectral data
– It also provides structural clues
Fragmentation Pattern
• Fragmentation occurs when the high-energy cation radical
breaks down by spontaneous cleavage of a chemical bond
forming:
– a carbocation (the fragment with the positive charge)
– a neutral radical (the other fragment)
Positive charge goes to fragments that best can stabilize it
– Stable carbocation is formed
Mass Spectral Fragmentation of Hexane
• Hexane (m/z = 86 for parent) has peaks at m/z = 71,
57, 43, 29
Mass Spectral Fragmentation of Hexane
• Hexane fragments as follows:
Practice Problem: methylcyclohexane or ethylcyclopentane?
M+ - CH2CH3 = 69
M+ = 98
M+ - CH3 = 83
M+ = 98
Practice Problem: Two mass spectra are shown. One spectrum
corresponds to 2-methyl-2-pentene; the other,
to 2-hexene. Which is which? Explain.
M+ - CH3 = 69
M+ = 84
M+ - CH2CH3 = 55
M+ = 84
4.
Mass Spectral Behavior of Some
Common Functional Groups
• Mass-spectral fragmentations are usually complex
and difficult to interpret.
• However, there are some distinguishing features of
several common functional groups
– Functional groups cause common patterns of cleavage in
their vicinity
Mass Spectral Cleavage Reactions of Alcohols
• Alcohols undergo
– alpha () cleavage (at the bond next to the C-OH) and
– dehydration (loss of H-OH) to give C=C
neutral radical
alkene radical cation
m/z = (M+ -18)
Mass Spectral Cleavage Reactions of Amines
• Amines undergo
– alpha () cleavage (at the bond next to the C-N),
generating an alkyl radical and a N-containing cation
alkyl radical
Mass Spectral Cleavage of Carbonyl compounds
• Ketones and aldehydes undergo:
– McLafferty rearrangement
• a H on a carbon three atoms away from the carbonyl group (C=O)
is transferred to the O of the C=O,
• a C-C bond is broken, and
• a neutral alkene fragment is produced
– alpha () cleavage
• at the bond between the C=O and the neighboring C
• Ketones and aldehydes undergo:
– McLafferty rearrangement
– alpha () cleavage
neutral radical
Practice Problem: Identify fragments for 2-methyl-3-pentanol MS
Practice Problem: What are the masses of the charged fragments
produced in the following cleavage pathways?
a) Alpha cleavage of 2-pentanone (CH3COCH2CH2CH3)
b) Dehydration of cyclohexanol (hydroxycyclohexane)
c) McLafferty rearrangement of 4-methyl-2-pentanone
[CH3COCH2CH(CH3)2]
d) Alpha cleavage of triethylamine [(CH3CH2)3N]
Practice Problem: List the masses of the parent ion and of
several fragments you might expect to find in
the mass spectrum of the following molecule
(red = O)
5.
Spectroscopy and the Electromagnetic
Spectrum
• Unlike mass spectrometry, infrared (IR), ultraviolet
(UV) and nuclear magnetic resonance (NMR)
spectroscopies:
– are nondestructive
– involve interaction of molecules with electromagnetic
energy rather than with high-energy electron beam
The Electromagnetic Spectrum
• The electromagnetic spectrum is the range of electromagnetic
energy, including IR, UV and visible radiation
• The electromagnetic spectrum covers a continuous range of
wavelengths and frequencies, radio waves to g rays
High n
Low l
Low n
High l
• Electromagnetic radiation has dual behavior:
– It behaves as a particle (called a photon)
– It behaves as an energy wave
• Electromagnetic energy is transmitted only in discrete
amounts called quanta.
• Electromagnetic waves are characterized by:
– a wavelength (l)
– a frequency (n)
– an amplitude
Wavelength, Frequency and Amplitude
• Wavelength (l) – is the distance from one wave maximum to the next
• Frequency (n) - is the # of waves that pass by a fixed point per unit
time (s-1 or Hz)
• Amplitude - is the height of a wave, measured from midpoint to peak
Wavelength x Frequency = Speed
l (m)
l=
x
c
n
n (s-1)
n=
=
c
c
l
Speed of light: Cvacuum = 3.00 x 108
m/s
• The Planck equation gives:
e = hn =
hc
l
where e = Energy of 1 photon (1 quantum)
h = Planck’s constant (6.62 x10-34J.s)
n = Frequency (s-1)
l = Wavelength (m)
c = Speed of light (3.00 x 108 m/s)
• Radiant energy is proportional to its frequency and
inversely proportional to its wavelength
• The Planck equation can be rewritten:
E = NA e =
NA hc
l
=
1.20 x 10-4 kJ/mol
l
where E = Energy of Avogadro’s number of photons
NA= Avogadro’s number
e = Energy of 1 photon (1 quantum)
h = Planck’s constant (6.62 x10-34J.s)
c = Speed of light (3.00 x 108 m/s)
l = Wavelength (m)
Absorption Spectrum
• Organic compounds exposed to electromagnetic radiation
can absorb energy of certain wavelengths but transmit
energy of other wavelengths
– They can absorb photons of specific energies (wavelengths or
frequencies)
• Changing wavelengths to determine which are absorbed
and which are transmitted produces an absorption
spectrum
• Energy absorbed is distributed internally in a distinct and
reproducible way
• An absorption spectrum shows the wavelength on the x-axis and
the intensity of the various energy absorptions expressed in %
transmittance on the y-axis.
Ethyl alcohol
CH3CH2OH
Practice Problem: Which has higher energy, infrared radiation
with l = 1.0 x 10-6 m or an X ray with
l = 3.0 x 10-9 m?
Practice Problem: Which has higher energy, radiation with
n = 4.0 x 109 Hz or radiation with l = 9.0 x 10-6
m?
Practice Problem: Calculate the energies of each of the following
kinds of radiation using the relationships
E=
1.20 X 10-4 kJ/mol .
l (m)
and
n= c .
l
a) A gamma ray with l = 5.0 x 10-11 m
b) An X-ray with l = 3.0 x 10-9 m
c) Ultraviolet light with n =6.0 x 1015 Hz
d) Visible light with n =7.0 x 1014 Hz
e) Infrared radiation with l = 2.0 x 10-5 m
f) Microwave radiation with n =1.0 x 1011 Hz
6.
Infrared Spectroscopy of Organic
Molecules
• The infrared (IR) region is lower in photon energy
than visible light
– Only 2.5 10-6 m to 2.5 10-5 m region is used by
organic chemists for structural analysis
Absorption Spectrum
• IR energy in a spectrum is usually measured as
wavenumber
~
• Wavenumber (n) is the inverse of wavelength
is proportional to frequency
is expressed in cm-1
1
Wavenumber (cm-1) =
l (cm)
• Specific IR absorbed by organic molecule is related to its
structure
Infrared Energy Modes
• Molecules are in constant motion (i.e bond stretching,
contracting, bending…)
– Their energy is quantized
Infrared Energy Modes
• Combinations of atomic movements, such as bending and
stretching of bonds between groups of atoms, are called
“normal modes”
– IR energy absorption corresponds to specific modes
Infrared Energy Modes
• When a molecule is irradiated with electromagnetic
radiation, energy is absorbed if the frequency of the
radiation matches the frequency of the vibration.
– Energy absorption increases amplitude for the vibration
Infrared Energy Modes
• IR energy - is characteristic of the atoms in the group and
their bonding
- corresponds to the amount of energy needed
to increase the amplitude of specific molecular
vibrations
Practice Problem: Because IR absorptions can be expressed
either in micrometers or in wavenumbers, it’s
useful to be able to interconvert between units.
Do the following conversions:
a) 3.10 mm to cm-1
b) 5.85 mm to cm-1
c) 2250 cm-1 to mm
7.
Interpreting Infrared Spectra
• Most functional groups absorb at about the same
energy and intensity independent of the molecule in
which they are.
• Characteristic IR absorptions
– can be used to confirm the presence of a functional group in
a molecule
– are listed in Table 12.1
Fingerprint Region of Infrared Absorption Spectrum
• IR spectrum has a lower energy region characteristic
of molecule as a whole known as “fingerprint” region.
– Its range goes from 1500 cm-1 to 400 cm-1
Hexane
1-hexene
1-hexyne
Regions of Infrared Absorption Spectrum
• 4000-2500 cm-1 N-H, C-H, O-H (stretching)
– 3300-3600 N-H, O-H
– 3000 C-H
• 2500-2000 cm-1 CC and CN (stretching)
Regions of Infrared Absorption Spectrum
• 2000-1500 cm-1 double bonds C=O, C=C C=N (stretching)
– C=O 1680-1750
– C=C 1640-1680 cm-1
• Below 1500 cm-1 “fingerprint” region
Differences in Infrared Absorptions
• Molecules vibrate and rotate in normal modes, which are
combinations of motions
– These are related to force constants
• Bond stretching dominates higher energy (frequency) modes
Differences in Infrared Absorptions
• Light objects connected to heavy objects vibrate fastest (at
higher frequencies): C-H, N-H, O-H > C-O, C-N
• For two heavy atoms, stronger bond requires more energy
(higher frequency): CC, CN > C=C, C=O, C=N > C-C,
C-O, C-N, C-X
Practice Problem: Refer to Table 12.1, and make educated
guesses about what functional groups the
following molecules might contain:
a) A compound with a strong absorption at 1710 cm-1
b) A compound with a strong absorption at 1540 cm-1
c) A compound with strong absorptions at 1720 cm-1
and 2500-3100 cm-1
Practice Problem: How might you use IR spectroscopy to
distinguish between the following pairs of
isomers?
a) CH3CH2OH and CH3OCH3
b) Cyclohexane and 1-hexene
c) CH3CH2CO2H and HOCH2CH2CHO
8.
Infrared Spectra of Hydrocarbons
• C-H, C-C, C=C, CC have characteristic peaks
Example: Hexane
Alkenes
Example: 1-hexene
3100
1660
Alkynes
(Terminal alkyne)
Example: 1-hexyne
2100
3300
Practice Problem: The IR spectrum of phenylacetylene is shown
below. What absorption bands can you
identify?
9.
Infrared Spectra of Some Common
Functional Groups
• Spectroscopic behavior of functional groups is
discussed in later chapters
• Brief summaries are presented here
Alcohols
Example: Cyclohexanol
Amines
Example: Cyclohexylamine
Aromatic Compounds
Example: phenylacetylene
Ring bonds
1450-1600 cm-1
Carbonyl Compounds
• Strong, sharp C=O peak at 1670 to 1780 cm-1
• The exact position of absorption within the range is
characteristic of each type of carbonyl compound.
– It can often be used to identify aldehydes, ketones, and
esters.
Aldehydes
• 1730 cm-1 in saturated aldehydes
• 1705 cm-1 in aldehydes next to double bond or aromatic
ring
Example: Phenylacetaldehyde
C=O
1725 cm-1
Ketones
• 1715 cm-1 in six-membered ring or acyclic ketones
• 1750 cm-1 in five-membered ring ketones
• 1690 cm-1 in ketones next to a double bond or an aromatic
ring
Example: cyclohexanone
1715 cm-1
Ring bonds
1450-1600 cm-1
Esters
• 1735 cm-1 in saturated esters
• 1715 cm-1 in esters next to aromatic ring or a double bond
Practice Problem: Where might the following compounds have IR
absorptions?
Practice Problem: Where might the following compound have IR
absorptions? (Red = O, blue = N)
Problem 1: Cyclohexane or Cyclohexene?
Problem 2: Propose structure(s) for unknown hydrocarbon
Problem 3: Propose structure(s) for unknown hydrocarbon
Chapter 12
Chromatography: Purifying Organic Compounds
• Chromatography – is a process that separates compounds
using adsorption and elution
– Mixture is dissolved in a solvent (mobile phase) and placed into
a glass column of adsorbent material (stationary phase)
– Solvent or mixtures of solvents passed through
– Compounds adsorb to different extents and desorb differently in
response to appropriate solvent (elution)
– Purified sample in solvent is collected from end of column
– Can be done in liquid or gas mobile phase
Principles of Chromatography
• Stationary phase is alumina (Al2O3) or silica gel
(hydrated SiO2)
• Solvents of increasing polarity are used to elute and
more strongly adsorbed species (polar) migrate more
slowly
• Polar species adsorb most strongly to stationary phase
– For examples, alcohols adsorb more strongly than alkenes
High-Pressure (or High-Performance) Liquid Chromatography (HPLC)
• More efficient and complete separation than ordinary LC
• Coated silica microspheres (10-25 µm diameter) in
stationary phase
• High-pressure pumps force solvent through tightly
packed HPLC column
• Detector monitors eluting material
HPLC of Pesticide Mixture
• HPLC analysis of a mixture of 14 pesticides, using
acetonitrile/water as the mobile phase
Chapter 12