File
advertisement

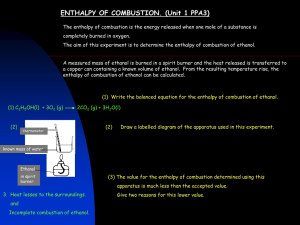
A Comparison of the Efficiency of Starch-Ethanol From Maize Kernels And Cellulosic-Ethanol From Corncobs as Bio-fuels 4S 2012-2013 I. Background and Significance II. Objectives III. Hypothesis IV. Methodology V. Data VI. Analysis VII. Recommendations VIII.Conclusion Background and Significance •Other than being a renewable source of energy, biofuels are also considered more environmentally friendly than petroleum. •This is mainly because of the presence of an additional oxygen atom in ethanol, which reduces carbon monoxide Background and Significance • A first-generation bio-fuel comes from seeds, grains, or sugars. Ethanol or butanol is fermented from starch or sugar. • Second-generation bio-fuel is produced from lignocellulosic biomass, which means low-cost, nonedible feedstock can be used. Background and Significance • A first-generation bio-fuel competes with crops used for food. Second generation bio-fuels can be made using crop wastes such as husks and cobs. Background and Significance • Corn is one of the most abundant biomass in the Philippines. • Estimated output of for the whole of 2010: 6.8 MMT Objectives 1) Identify which material, lignocellulosic biomass or starch-laden biomass, will produce more ethanol when equal samples (by mass) are fermented by Saccharomyces cerevisiae (yeast). Objectives 2) Compare the efficiency between the bio-fuel made from the ethanol extracted from the fermented lignocellulosic material (corn cobs) and the bio-fuel made from the ethanol extracted from the starch-laden material (maize kernels) when combusted and identify which between them will serve as the more efficient petroleumgasoline substitute. Objectives 3) Compare the efficiency of the bio-fuels made from the extracted ethanol samples with the commercialized petroleum-gasoline when combusted. Hypothesis 1) If the amount of lignocellulosic biomass to be fermented is equal (by mass) to the amount of starch-laden biomass to be fermented, then the amount of cellulosic ethanol extracted will be equal (by volume) to the amount of starch ethanol extracted. Hypothesis 2) If the bio-fuel made from the cellulosic ethanol, and the bio-fuel made from the starch were combusted to test and compare their respective efficiencies as petroleum-gasoline substitutes, then the efficiency of the bio-fuel made from the cellulosic ethanol samples will be equal to, or greater than, the efficiency of the bio-fuel made from the starch ethanol samples. Hypothesis 3) If the bio-fuel made from the cellulosic ethanol samples, and the commercialized petroleumgasoline were combusted to test and compare their respective efficiencies as fuel, then the efficiency of the bio-fuel made from the cellulosic ethanol samples will be equal to, or greater than, the efficiency of commercialized petroleumgasoline. Methodology A) Pre-treatment B) Fermentation C) Distillation D) Combustion Pre-treatment Pre-treatment Pre-treatment Fermentation Distillation Distillation Distillation Combustion Combustion Data Data Data Analysis For this experiment, 26.7% of the lignocellulose and 30.5% of the starch laden biomass were converted into ethanol through the various processes of breaking down through physical and chemical means, and by adding yeast (Saccharomyces cerevisia),ethanol was produced and extracted through distillation. Analysis The gasoline mixed with ethanol produced results with equal torque, rpm and speed, while pure gasoline slightly exceeded the performance of the bio-fuels. Because ethanol has a lower density as to pure gasoline, there is less fuel to be burned if equal volumes were used. As such, bio-fuels will give of less heat energy that will affect the amount of energy the engine can give off. Recommendations 1) Better Pre-treatment 2) Better Distillation 3) Test concentration of ethanol Conclusion We therefore conclude that production of a second generation bio-fuel requires more biomass than the biomass needed for the production of a first generation bio-fuel. However, second generation bio-fuels perform just as efficiently as first generation bio-fuels when combusted in equal concentrations in an internal combustion engine.