幻灯片 1
advertisement
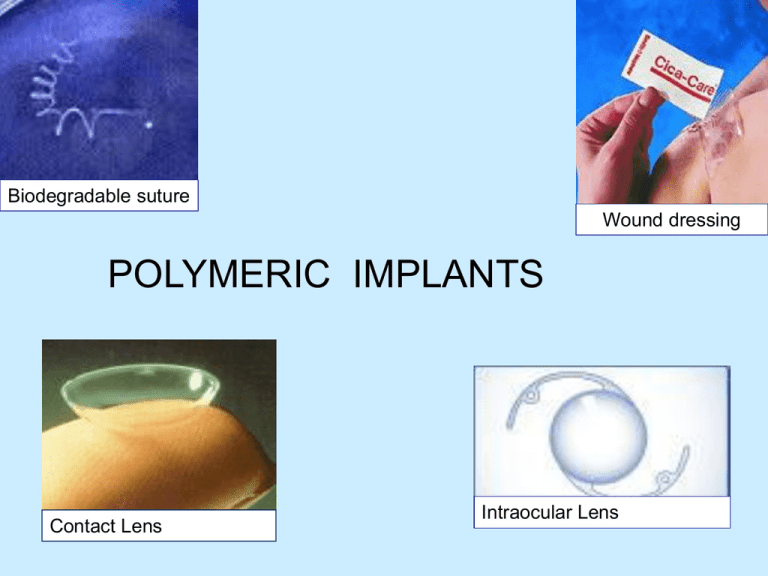
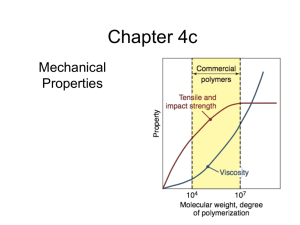
Biodegradable suture Wound dressing POLYMERIC IMPLANTS Contact Lens Intraocular Lens Some Commonly Used Polymers Material Applications Silicone rubber Catheters, tubing Dacron Vascular grafts Cellulose Dialysis membranes Poly(methyl methacrylate) Intraocular lenses, bone cement Polyurethanes Catheters, pacemaker leads Hydogels Opthalmological devices, Drug Delivery Collagen (reprocessed) Opthalmologic applications, wound dressings Polymer Devices Advantages: Disadvantages: Examples: Some joint replacement articulating surfaces Spinal cages Biodegradable bone plates for low load regions Biodegradable sutures Hip joint Spinal cage for spine fusion Bone plates Mechanical Properties: Why is important to study for all biomaterials? Determines how well it will work (or not work) for a given device. One major factor is the modulus of the material. metal polymer polymer Toe implant ______________ hydrogel ____________ Polymers • Terminology: – copolymer: polymers of two mer types • random · · ·-B-A-B-A-B-B-A-· · · • alternating· · ·-A-B-A-B-A-B-A-· · · • block · · ·-A-A-A-A-B-B-B-· · · – heteropolymer: polymers of many mer types COPOLYMER Polymers Structure Linear Branched Crosslinked Synthetic Polymers Biodegradable Synthetic Polymers • Poly(alkylene ester)s • PLA, PCL, PLGA • Poly(aromatic/aliphatic ester)s • Poly(amide-ester)s • Poly(ester-urethane)s • Polyanhydrides • Polyphosphazenes Biostable Polymers • Polyamides • Polyurethanes • Polyethylene • Poly(vinylchloride) • Poly(hydroxyethylmethacrylate) • Poly(methylmethacrylate) • Poly(tetrafluoroethylene) • Poly(dimethyl siloxane) • Poly(vinylalcohol) • Poly(ethylenglycol) Stimuli Responsive Poly(ethylene oxide-co-propilene oxide) Poly(methylvinylether) Poly(N-alkyl acrylamide)s Poly(phosphazone)s Polymers Bioinert Biodegradable Polymers Natural Synthetic Synthetic Biomaterials • POLYMERS: Silicones, Gore-tex (ePTFE), Polyethylenes (LDPE,HDPE,UHMWPE,) Polyurethanes, Polymethylmethacrylate, Polysulfone, Delrin • Uses: Orthopedics, artificial tendons, catheters, vascular grafts, facial and soft tissue reconstruction • COMPOSITES: CFRC, self reinforced, hybrids • Uses: Orthopedics, scaffolds • HYDROGELS: Cellulose, Acrylic co-polymers • Uses: Drug delivery, vitreous implants, wound healing • RESORBABLES: Polyglycolic Acid, Polylactic acid, polyesters • Uses: sutures, drug delivery, in-growth, tissue engineering Polymers: Biomedical Applications • Polyethylene (PE) (C2H4)nH2 – five density grades: ultrahigh, high, low, linear low and very low density – UHMWPE and HDPE more crystalline – UHMWPE has better mechanical properties, stability and lower cost – UHMWPE can be sterilized Polymers: Biomedical Applications • UHMWPE: Acetabular caps in hip implants and patellar surface of knee joints. • HDPE used as pharmaceutical bottles, fabrics. • Others used as bags, pouches, tubes etc. Artificial Hip Joints (UHMWPE) http://www.totaljoints.info/Hip.jpg Polymers: Biomedical Applications • Polymethylmethacrylate (PMMA, lucite, acrylic, plexiglas) • (C5O2H8)n – acrylics – transparency – tough – biocompatible • Used in dental restorations, membrane for dialysis, ocular lenses, contact lenses, bone cements Intraocular Lens 3 basic materials - PMMA, acrylic, silicone Polymers: Biomedical Applications • Polyamides (PA, nylon) • PA 6 : [NH−(CH2)5−CO]n made from ε-Caprolactam – high degree of crystallinity – interchain hydrogen bonds provide superior mechanical strength (Kevlar fibers stronger than metals) – plasticized by water, not good in physiological environment • Used as sutures Polymers: Biomedical Applications • Polyvinylchloride (PVC) (monomer residue must be very low) – Cl side chains – amorphous, hard and brittle due to Cl – metallic additives prevent thermal degradation • Used as blood and solution bags, packaging, IV sets, dialysis devices, catheter, bottles, cannulae Polymers: Biomedical Applications • Polypropylene (PP) (C3H6)n – properties similar to HDPE – good fatigue resistance • Used as syringes, oxygenator membranes, sutures, fabrics, vascular grafts • Polyesters (polymers which contain the ester functional group in their main chain) • PET (C10H8O4)n – hydrophobic (beverage container PET) – molded into complex shapes • Used as vascular grafts, sutures, heart valves, catheter housings Polymers: Biomedical Applications • Polytetrafluoroethylene (PTFE, teflon) (C2F4)n – low coefficient of friction (low interfacial forces between its surface and another material) – very low surface energy – high crystallinity – low modulus and strength – difficult to process • catheters, artificial vascular grafts Polymers: Biomedical Applications • Polyurethanes – block copolymer structure – good mechanical properties – good biocompatibility • tubing, vascular grafts, pacemaker lead insulation, heart assist balloon pumps Polyurethanes A urethane has an ester group and amide group bonded to the same carbon. Urethanes can be prepare by treating an isocyanate with an alcohol. O RN C O an isocyanate + ROH RNH C OR an alcohol a urethane Polyurethanes are polymers that contain urethane groups. CH3 O C N N C O + HOCH2CH2OH ethylene glycol toluene-2,6-diisocyanate O C NH CH3 O O CH3 NH C OCH2CH2O C NH O O NH C OCH2CH2O C n a polyurethane Synthetic vascular grafts from W.L.Gore Useful Definitions Biodegradable Undergoes degradation in the body - Degradation: _____________________________ - Degradation products are harmless and can be secreted naturally water Lactic acid PLLA bone plates Polymers: Biomedical Applications • Rubbers – latex, silicone – good biocompatibility • Used as maxillofacial prosthetics Biomedical polymer Poly(ethylene) (PE) Low density (LDPE) High density (HDPE) Ultra high molecular weight (UHMWPE) Application Bags, tubing Nonwoven fabric, catheter Orthopedic and facial implants Poly(methyl methacrylate) (PMMA) Intraocular lens, dentures, bone cement Poly(vinyl chloride) (PVC) Blood bags, catheters, cannulae Poly(ethylene terephthalate) (PET) Artificial vascular graft, sutures, heart valves Poly(esters) Bioresorbable sutures, surgical products, controlled drug release Poly(amides) (Nylons) Catheters, sutures Poly(urethanes) (PU) Coat implants, film, tubing Table The clinical uses of some of the most common biomedical polymers relate to their chemical structure and physical properties. Hydrogels • Water-swollen, crosslinked polymeric structure produced by reactions of monomers or by hydrogen bonding • Hydrophilic polymers that can absorb up to thousands of times their dry weight in H2O • Three-dimensional insoluble polymer networks Applications of Hydrogels • • • • • • • • Soft contact lenses Pills/capsules Bioadhesive carriers Implant coatings Transdermal drug delivery Electrophoresis gels Wound healing Chromatographic packaging material Types of Hydrogels • Classification – Method of preparation • Homo-polymer, Copolymer, Multi-polymer, Interpenetrating polymeric – Ionic charge • Neutral, Catatonic, Anionic, Ampholytic – Physical structure • Amorphous, Semi-crystalline, Hydrogen-bonded Types of Gelation • Physical , Chemical ژلهاي شدن فيزيكي :زنجيرهاي پليمر از طريق واكنشهاي يوني ،پيوند هيدروژني ،درهم گره خوردن مولكولي يا از راه طبيعت آبگريزي ماده اتصال مييابند. ژلهاي شدن شيميايي :زنجيرهاي هيدروژل با پيوند كوواالنت به يكديگر متصل شدهاند. در اين فرآيند ،روشهايي نظير تابش، افزودن اتصالدهندههاي عرض ي شيميايي و كار تركيبات واكنشگر چند منظوره به ميروند. Types of Hydrogels • Natural Polymers – Dextran, Chitosan, Collagen, Alginate, Dextran Sulfate, . . . – Advantages • • • • • Generally have high biocompatibility Intrinsic cellular interactions Biodegradable Cell controlled degradability Low toxicity byproducts – Disadvantages • Mechanical Strength • Batch variation • Animal derived materials may pass on viruses Types of Hydrogels • Synthetic Polymers – PEG-PLA-PEG, Poly (vinyl alcohol) – Advantages • Precise control and mass produced • Can be tailored to give a wide range of properties (can be designed to meet specific needs) • Low immunogenecity • Minimize risk of biological pathogens or contaminants – Disadvantages • Low biodegradability • Can include toxic substances • Combination of natural and synthetic – Collagen-acrylate, P (PEG-co-peptides) Properties of Hydrogels • Swelling properties influenced by changes in the environment – pH, temperature, ionic strength, solvent composition, pressure, and electrical potential • Can be biodegradable, bioerodible, and bioabsorbable • Can degrade in controlled fashion Properties of Hydrogels • Pore Size • Fabrication techniques • Shape and surface/volume ratio • H2O content • Strength • Swelling activation Advantages of Hydrogels • Environment can protect cells and other substances (i.e. drugs, proteins, and peptides) • Timed release of growth factors and other nutrients to ensure proper tissue growth • Good transport properties • Biocompatible • Can be injected • Easy to modify Disadvantages of Hydrogels • Low mechanical strength • Hard to handle • Difficult to load • Sterilization Why Hydrogels ?: Tissue Engineering • • • • • Biocompatible H2O content Sterilizibilty Ease of use High mechanical Strength • Surface to volume ratio • Good cell adhesion • High nutrient transport Why Hydrogels?: Cell Culture Systems • Biocompatible substrate – Non-toxic and have no immunological responses • Cytoarchitecture which favors cell growth – Flexibility for cells to rearrange in 3-D orientation – Seeded with appropriate growth and adhesion factors – Porosity (i.e. channels for nutrients to be delivered) Why Hydrogels?: Cell Culture Systems • Mimic cytomechanical situations – 3-D space provides balanced cytoskeleton forces – Dynamic loading to promote cell growth • Flexibility – Provide scaffold for various cells • Consistent, reproducible and easy to construct Why Hydrogels?: Drug Delivery • • • • • • • • Safe degradation products Biocompatible High loading with ensured molecule efficacy High encapsulation Variable release profile Stable Inexpensive High quality • Hydrogels are network polymers that swell through a variety of mechanisms in an aqueous environment • Environment controls mechanisms of swelling: – pH, ionic strength, solvent composition, pressure and even electric fields • Applications in medicine, engineering, and biology Chitosan • Chitosan (2-amino-2deoxy(1→4)-β-D-glucopyranan), a polyaminosaccharide, • obtained by alkaline deacetylation of chitin (the principal component of living organisms such as fungi and crustacea). Chitosan’s key properties: • 1) biocompatibility • 2) nonantigenicity • 3) nontoxicity (its degradation products are known natural metabolites) • 4) the ability to improve wound healing/or clot blood • 5) the ability to absorb liquids and to form protective films and coatings, and • 6) selective binding of acidic liquids, thereby lowering serum cholesterol levels. Alginate Guluronic acid Mannuronic acid These products are produced from naturally occurring calcium and sodium salts of alginic acid found in a family of brown seaweed. Alginates are rich in either mannuronic acid or guluronic acid, the relative amount of each influence the amount of exudate absorbed and the shape the dressing will retain. • فصل 10و 11کتاب زیستمواد ،اندامهای مصنوعی و مهندس ی بافت