mixture
advertisement

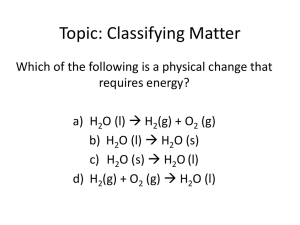
Do Now: Write down three examples of physical changes. Music: Rush – Tom Sawyer Book Section: 2.2 Classifying Matter A pure substance is a single component with unique chemical properties. Ex: ethanol, water, any element or compound A mixture is a blend of two or more components. A mixture can either be classified as homogeneous or heterogenous. Classifying Matter Homogeneous mixtures – can not tell components from each other. Also called solutions Examples: broth, plain yogurt, salt water Heterogenous mixtures – can tell the components apart Exampes: salad, Italian salad dressing, oil & vinegar Demo: Oil/Vinegar, sugar water Classification of Matter Classification of Matter Classification of Matter Classification of Matter Classification of Matter Separation of Mixtures Distillation uses differences in boiling points to boil and recondense liquids from each other. Equipment Show & Tell: Condenser, Distiller. Separation of Mixtures In filtration, solid substances are separated from liquids and solutions. Show & Tell: Filtration Setup Separation of Mixtures Chromatography separates substances on the basis of differences in solubility in a solvent. Heterogenous or Homogenous? Food coloring Ice cubes in liquid water Mouthwash Mashed, unpeeled potatoes Heterogenous or Homogenous? Food coloring - homogenous Ice cubes in liquid water - heterogenous Mouthwash - homogenous Mashed, unpeeled potatoes - heterogenous Homework 1-6 Due Tomorrow! This week: Thursday – Elements and Compounds (2.3) – HW 1-6 Due Friday – Chemical Reactions & Conservation of Mass (2.4) – HW 1-7 Due Unit 1 Test – Thursday, September 16