Homogeneous mixtures
advertisement

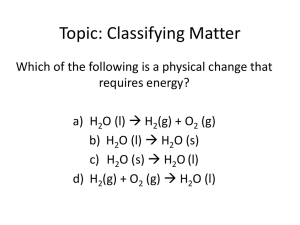
Justin Bieber says: "We're classifying matter" Matter is anything that has mass and takes up space. Matter ca be made up of particles called atoms. It can exist as a solid, liquid, gas, or plasma. All matter has both physical and chemical properties. Physical Properties ·Can be observed without changing the identity of the matter. What's it mean by "identity"? The identity is the molecular formula of the matter. It is the stuff that makes up the matter. Water is H2O whether it's a solid, liquid, or a gas Examples: melting, freezing, boiling, solubility and density Density ·Describes the relationship between mass and volume m v The density of a solid is expressed in The density of a liquid is expressed in g/ml Remember: 1 cm3 = 1ml g/cm3 Chemical Properties ·Describe a substance based on its ability to change into a new substance with different properties. What does this mean? It means that when observing this property it changes the identity of the matter. When or 2 or more substances react they change into new substance with new properties Examples: Flammability, reactivity, rusting, tarnishing, patina Characteristics Properties A property that is unique to each substance. They can be used to identify a substance. It does NOT depend on the amount of the material or the element. They include freezing, boiling, or melting points, density, color, reactivity, and solubility. Physical and Chemical Changes A physical change affects one or more physical properties. It does not change its identity. ·Melting gold or butter ·Breaking a pencil in half ·Any change of state (boiling, freezing, evaporating, etc.) ·A chemical change occurs when one or more substance change into new substances with different properties. Burning (flammability) Reactivity A chemical change cannot be undone by physical means but they can sometimes be undone by another chemical reaction link Physical or Chemical Changes Physical and Chemical A candle? Elements, Compounds, and Mixtures http://my.hrw.com/sh/hp1/0030724929/student/ch04/sec01/qc01/hp104_01_q01fs.htm An element is a pure substance that cannot be separated into simpler substances by physical and chemicals means. Elements are identified by the nubmer of protons in the nucleus 0 A compound is a pure substance that is composed of two or more elements that are chemically combined in a specific ratio. http://my.hrw.com/sh/hp1/0030724929/student/ch04/sec02/qc01/hp104_02_q01fs.htm Pure Substance is a substance in which there is only one type of material Example: elements or compounds Mixtures are a combination of 2 or more substances that are NOT chemically combined. They can be separated by chemical means. Chicken Noodle soup is an example because the pieces of chicken can be separated from the soup by physical means. Pizza is a mixture as well. Can you have different types of mixtures? Yes!!!! But how are we going to classify them? Scientists separate mixtures into 2 categories: Homogeneous or Heterogeneous Homogeneous Mixtures Homogeneous mixtures are combinations of matter where the particles are too small to be seen and they are spread out evenly throughout the mixture. Light can pass through a homogeneous mixture because the particles are too small to scatter the light. Solutions are homogeneous mixtures---a mixture that appears to be a single substance but it's really made of 2 or more materials distributed evenly amongst each other. Solutions are made up of 2 parts: The solute--the stuff dissolved The solvent- the stuff the solute is dissolved in he solute is dissolved in. Solubility is the ability to dissolve a substance into a solution. For example: sugar and salt can be dissolved into water so they have solubility. All solutions have solubility and concentrations! Concentration is the actual amount of solute dissolved in the solvent. It is measured in g/ml. Types of solutions: ·gas in a gas (air) ·gas in a liquid (soda) ·liquid in a liquid (hydrogen peroxide) ·solid in a liquid (kool-aid) ·solid in a solid (metal alloys) Heterogeneous mixtures Heterogeneous mixtures are mixtures that contain particles that are large enough to be seen and too heavy to remain suspended in the solvent. For example: Vinegarettes and salad dressings, sand and gravel mixtures, a snow globe Suspensions are heterogeneous mixtures in which the particles are so large that they eventually will settle out (fall to the bottom) Sand and water, salad dressings, muddy water The particles are large enough to scatter the light Matter Mixture Pure Substance Elements Compounds Homogeneous Solutions Heterogeneous Suspensions 3 Major categories of Elements http://my.hrw.com/sh/hp1/0030724929/student/ch04/sec01/qc03/hp104_01_q03fs.htm Metal--malleable (can be flattened into sheets), ductile (can be made into wires), shiny, good conductors of heat and electricity. Nonmetal-- dull, poor conductors of thermal or electrical energy (easily break) Metalloid--semiconductors, have properties of both metals and nonmetals, only conduct electrical energy at high temperatures.