Metal Reactivity Series Lab: Single Displacement Reactions
advertisement

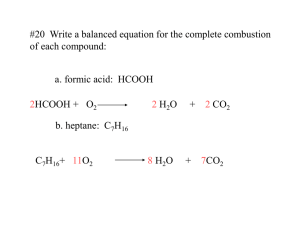
From single displacement reactions it is easy to see some metals are more reactive than others. For instance when iron combines with copper sulfate the iron "steals" the sulfate from the copper. This means if an activity series was created with the more reactive metal on top and the less reactive metal on bottom the result would be: Iron Copper You will now perform a series of single displacements using metals and solutions containing metal ions. This will enable you to create your own reactivity series. Reaction Plate Place a small amount CuSO4 in 4 of the wells. Repeat the same step with solutions of Iron(II) chloride, zinc nitrate, aluminium nitrate, magnesium chloride CuSO4 FeCl3 ZnNO3 Al(NO3)2 MgCl2 CuSO4 FeCl2 ZnNO3 Al(NO3)2 MgCl2 Al, Mg, Cu in each of the wells but don’t place metals with solutions which are the same. CuSO4 FeCl3 Zn(NO3)2 Al(NO3)3 MgCl2 Fe Zn Al Mg Cu Now observe the wells using a dissecting microscope to detect any evidence of chemical change. Construct an activity series based on the empirical evidence. Fe Zn Al Mg Cu Zn Fe Al Mg Cu Cu Zn Fe Al Mg Mg Cu Zn Fe Al Al Mg Cu Zn Fe Write a lab report. In the conclusion include balanced chemical equations for all observed single displacement reactions. There are more than 20 possible chemical reactions. For metals with multiple oxidation states write all possible reactions. If no reaction occurs write the reactants with an arrow and NR (no reaction) as the product. If Cu doesn’t react with MgCl2(aq) write: Cu + MgCl2 ------> NR follow this sequence: CuSO4 FeCl3 Zn(NO3)2 Al(NO3)3 MgCl2 Fe Zn Al Mg Cu Products of Reactions CuSO4 FeCl 3 Zn(NO3)2 Al(NO3)3 MgCl 2 Fe Zn Al Mg Cu FeSO4 + Cu Cu + ZnSO4 Cu + Al2(SO4)3 Cu + MgSO4 x Fe + ZnCl2 Fe + AlCl3 Fe + MgCl2 Zn + Al(NO3)2 Zn + Mg(NO3)2 x Al + Mg(NO3)2 x x x Products of Reactions CuSO4 FeCl 3 Zn(NO3)2 Al(NO3)3 MgCl 2 Fe Zn Al Mg Cu FeSO4 + Cu Cu + ZnSO4 Cu + Al2(SO4)3 Cu + MgSO4 x Fe + ZnCl2 Fe + AlCl3 Fe + MgCl2 Zn + Al(NO3)2 Zn + Mg(NO3)2 x Al + Mg(NO3)2 x x x Mg Al Zn Fe Cu