Lecture 11
advertisement

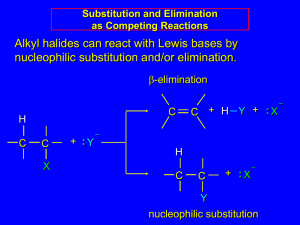
Heterolytic nucleophilic substitution:SN1 and SN2: halogenoalkanes with nucleophiles Heterolytic nucleophilic substitution:SN1 and SN2: halogenoalkanes with nucleophiles 1 Heterolytic nucleophilic substitution:SN1 and SN2: halogenoalkanes with nucleophiles When carbon is bonded This can result in to a more complete electronegative atom either heterolytic breakage of the bond. This atom applies an inductive effect on the electrons in the bond, pulling the electrons towards it. or This can result in charge separation along the bond. If the bond breaks heterolytically, two ions, a halide and a carbocation, are made. Charge separation creates an electron deficient carbon atom which is a reactive site with a center of positive charge. 2 Heterolytic nucleophilic substitution:SN1 and SN2: halogenoalkanes with nucleophiles These two possibilities lead to two different reaction mechanisms: SUBSTITUTION BY A NUCLEOPHILE; FIRST ORDER KINETICS: SN1 Stage 1 The carbon-halogen bond breaks heterolytically. Stage 2 The carbocation is attacked by a nucleophile. This step is very fast, so the rate determining step is stage 1. The rate of stage 1 depends only on the concentration of halogen compound, so the kinetics are first order. 3 Heterolytic nucleophilic substitution:SN1 and SN2: halogenoalkanes with nucleophiles These two possibilities lead to two different reaction mechanisms: SUBSTITUTION BY A NUCLEOPHILE; FIRST ORDER KINETICS: SN1 Stage 3 In the product, the halogen atom has been substituted by the nucleophile so the result is a substitution reaction. Because the carbocation is planar, it can be attacked equally well form either side. If the product has an asymmetric carbon atom, equal amounts of each optical isomer will be made. The product, although it contains optically active particles, contains equal amounts of each kind and so will not affect plane polarized light. It is called a racemic mixture.4 Heterolytic nucleophilic substitution:SN1 and SN2: halogenoalkanes with nucleophiles SUBSTITUTION BY A NUCLEOPHILE; SECOND ORDER KINETICS: SN2 Stage 1 Initial attack is by nucleophile on the electron deficient carbon atom. This initial step is the rate determining step and involves a collision between two particles, the halogen compound and the nucleophile, so the kinetics are second order. 5 Heterolytic nucleophilic substitution:SN1 and SN2: halogenoalkanes with nucleophiles SUBSTITUTION BY A NUCLEOPHILE; SECOND ORDER KINETICS: SN2 Stage 2 The intermediate has a high energy, which means that the overall rate of reaction is slow. The carbon being attacked is saturated and has a full outer shell because carbon cannot expand its outer shell. As the electron pair from the nucleophile moves in, the pair forming the sigma bond to the halogen moves away. This bond breaks heterolytically. 6 Heterolytic nucleophilic substitution:SN1 and SN2: halogenoalkanes with nucleophiles SUBSTITUTION BY A NUCLEOPHILE; SECOND ORDER KINETICS: SN2 Stage 3 In the product the halogen has been substituted by the nucleophile so the reaction is a substitution. The halide ion is a leaving group. The strength of the bond to the leaving group and the stability of the leaving group will affect the amount of product make. If the product has an asymmetric carbon atom, only one optical 7 isomer will be made. Heterolytic nucleophilic substitution:SN1 and SN2: halogenoalkanes with nucleophiles Words Words and Expressions heterolytic nucleophilic substitution halogenoalkane inductive effect carbocation substitute; substitution intermediate asymmetric optical isomer racemic 8 Heterolytic nucleophilic addition and addition/elimination:carnonyl compounds with hydrogen cyanide Heterolytic nucleophilic addition and addition/elimination: carbonyl compounds with hydrogen cyanide 9 Heterolytic nucleophilic addition and addition/elimination:carnonyl compounds with hydrogen cyanide Hydrogen cyanide adds across the double bond in a carbonyl compound, if there is a trace of the catalyst potassium cyanide present. 10 Heterolytic nucleophilic addition and addition/elimination:carnonyl compounds with hydrogen cyanide Stage 1 • Initial attack is by the lone pair of a nucleophile on an electron deficient carbon atom. The hydrogen cyanide is not a good nucleophile, because it does not have a prominent lone pair. But the cyanide ion does. • The positive reactive site on the carbon atom is due to the electron withdrawing or inductive effect of the more electronegative oxygen atom bonded to it. • The carbon atom is unsaturated. As the lone pair from the nucleophile moves in, the p pair between the carbon and the oxygen moves onto the oxygen at the same time. This movement of p electrons is called a 11 mesomeric(中介的) shift and is the heterolytic breaking of the p bond. Heterolytic nucleophilic addition and addition/elimination:carnonyl compounds with hydrogen cyanide Stage 2 The intermediate made has a four bonded carbon atom. This is the usual bonding for carbon and so the energy of the intermediate is low. A low activation energy means a fast reaction. If there is a good leaving group joined to the carbon, the lone pair on the oxygen may move back between the oxygen and carbon making the p bond again as the leaving group moves away. 12 Heterolytic nucleophilic addition and addition/elimination:carnonyl compounds with hydrogen cyanide Stage 3 The negatively charged oxygen atom made when the p pair moves onto it (becoming a lone pair) is a good base. This base deprotonates the protonated form of the nucleophile, here the hydrogen cyanide, replacing the cyanide ion which did the first attack. So the cyanide ion is a catalyst. Notice that the result of the whole reaction is the addition of a substance across the double bond. The hydrogen always ends up joined to oxygen. 13 Heterolytic nucleophilic addition and addition/elimination:carnonyl compounds with hydrogen cyanide Because the carbonyl group is planar, the nucleophile has an equal chance of attacking from above or below the plane of the molecule. This means that there are two possible products made in equal quantities. The two products can be optical isomers. As they are made in equal quantities, the product mixture is a racemate. 14 Umpolung & Benzoin Condensation • Umpolung (polarity inversion) is the chemical modification that reverses the polarity of a functional group. This modification allows secondary reactions of this functional group that would otherwise not be possible. • The concept was introduced by D. Seebach & E.J. Corey. • Benzoin condensation is a classical example of umpolung, in which the cyanide ion is an umpolung reagent. Umpolung: 极性翻转 Benzoin:安息香 Mechanism of Benzoin condensation 15 Heterolytic nucleophilic addition and addition/elimination:carnonyl compounds with hydrogen cyanide Words Words and Expressions addition/elimination add across a double bond cyanide withdraw mesomeric shift racemate Umpolung Benzoin condensation 16 Heterolytic electrophilic substitution: the nitration of benzene Heterolytic electrophilic substitution: the nitration of benzene 17 Heterolytic electrophilic substitution: the nitration of benzene Stage 1 Initial attack is by the p electrons of the aromatic system on the added electrophile. Only very strong electrophiles can react without the help of a catalyst. The usual catalyst is aluminum chloride and is called a Friedel-Crafts catalyst. Here the electron shifts happen one after another. Firstly, a p pair from the ring moves out. This movement of p electrons means that the delocalization stability of the ring is lost, so the process has a high activation energy. 18 Heterolytic electrophilic substitution: the nitration of benzene Stage 2 The deprotonation of the intermediate by some basic particle in the reaction mixture. Once the hydrogen has been removed, the delocalization stability can be restored. 19 Heterolytic electrophilic substitution: the nitration of benzene Stage 3 Production of a stable substitution product The nitro group has replaced or substituted for a hydrogen on the benzene ring. Aromatic stability is restored. 20 Heterolytic electrophilic addition: alkenes with halogens and hydrogen halides Heterolytic electrophilic addition: alkenes with halogens and hydrogen halides 21 Heterolytic electrophilic addition: alkenes with halogens and hydrogen halides Stage 1 Inititial attack is by p electrons of an unsaturated system on an electrophile. Sometimes the electrophile is induced or caused by the p system. Notice here that, unlike the mechanism above, the electron shifts happen at the same time. As the p pair moves towards the electrophile a s pair moves onto the far end of the bond in the 22 electrophile. This bond is breaking heterolytically. Heterolytic electrophilic addition: alkenes with halogens and hydrogen halides Stage 2 Nucleophilic attack on the intermediate. The intermediate is attacked from the other side by the nucleophile that was made in the heterolysis. p electrons are less strongly held, so the activation energy of the first step is low and the reaction goes fast in the cold. If the reaction is done in water, then the second attack might be by a 23 water molecule, which has nucleophilic properties. Heterolytic electrophilic addition: alkenes with halogens and hydrogen halides Stage 3 Production of trans-addition product The result of the whole reaction is addition across the double bond. Because the second attack is from the other side, the addition is always trans so that one part is joined on one side of the chain and the other on the other side of the chain. If the substance being added is HX, then the H goes onto the carbon with the most hydrogen atoms already bonded to it (Markovnikov’s 24 Rule). Heterolytic electrophilic addition: the nitration of benzene alkenes with halogens and hydrogen halides Words Words and Expressions nitration nitro group restore trans-addition 25 Homolytic substitution: a free radical photochemical chain reaction Homolytic substitution: a free radical photochemical chain reaction 26 Homolytic substitution: a free radical photochemical chain reaction Stage 1 Initiation: the production of free radicals. A chlorine molecule absorbs ultraviolet radiation. This energy makes the bond in the molecule break homolytically producing two free radicals. Because this reaction is started by light, it is a photochemical reaction. 27 Homolytic substitution: a free radical photochemical chain reaction Stage 2 Propagation(生长): the reaction between a free radical and a molecule to make another free radical. There are many possible steps. Because each step makes another reactive free radical, the reaction is a chain reaction. 28 Homolytic substitution: a free radical photochemical chain reaction Stage 3 Termination: the reaction between two free radicals making an unreactive molecule. Because any two free radicals can meet, this type of reaction always makes a mixture of products. 29 Hemolytic addition: A free radical polymerization reaction Homolytic addition: A free radical polymerization reaction 30 Hemolytic addition: A free radical polymerization reaction Stage 1 Initiation: the production of free radicals. At initiator, usually an organic peroxide, is heated. This energy makes the oxygen-oxygen bond in the molecule break homolytically producing two free radicals. 31 Hemolytic addition: A free radical polymerization reaction Stage 2 • Propagation: the reaction between a growing chain, which is a free radical, and a monomer molecule to make a longer growing chain radical. • There are many possible steps. • Because each step makes another reactive free radical, the reaction is a chain reaction. 32 Hemolytic addition: A free radical polymerization reaction Stage 3 Termination: two growing chains meet leading to the disappearance of the radical active sites. Because any two free radicals can meet, this type of reaction always makes a mixture of chains of different lengths. 33 Hemolytic addition: A free radical polymerization reaction Words Words and Expressions chain reaction initiation; propagation; termination photochemical reaction monomer polymer; polymerization 34