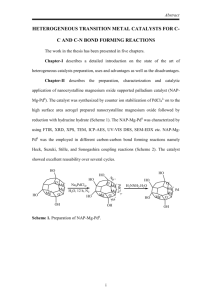
Couplings
advertisement

COMPANY PROFILE PARTNER FOR COOPERATION VUOS Cross coupling reactions Cross coupling reactions A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst with formation of a new carbon-carbon bond in the product. Cross couplings involve reactions 2between two different partners and homocouplings couple two identical partners, SYNTHESIA, AGROFERT HOLDING, a.s. 100% a.s. 100% VUOS developed all types of cross couplings reactions which are widely used in the production of organic specialties mainly for production of pharmaceutical intermediates and many others organic specialties. 2 VUOS – PARTNER FOR COOPERATION VUOS, a.s. SUZUKI REACTION The reaction is a coupling of an aryl- or vinyl-boronic acid with an aryl-halide catalyzed by a palladium complex. R HO B X catalyst R' R R' HO It is widely used to synthesize poly-olefins, styrenes, and substituted biphenyls. 3 Example AGROFERT HOLDING, a.s. Br 100% SYNTHESIA, O Ha.s. B OH COOH COOH I OH B OH C H3 3 VUOS – PARTNER FOR COOPERATION C H3 VUOS, a.s. 100% NEGISHI REACTION The cross coupling reaction involving an organozinc compound, an organic halide and a nickel or palladium catalyst creating a new carbon-carbon covalent bond. catalyst R X R' Zn X R R' It can be used for syntheses of wide range of organic. 4 Example CN C F3 ZnBr CN F 3C Br N H2 N H2 Br Zn Cl 4 VUOS – PARTNER FOR COOPERATION KUMADA REACTION The cross coupling is useful for generating carbon-carbon bonds by the reaction of a Grignard reagent and an organic halide. R R' M g X X catalyst R R' The reaction allows many synthetic applications, especially creaction of polythiophenes, useful in organic electronic devices. 5 Example O C 2H 5 C H3 M gBr Cl H 5C 2O C H3 H 5C 2O S Br Mg Br 5 VUOS – PARTNER FOR COOPERATION O C 2H 5 S HIYAMA REACTION The reaction is a palladium-catalyzed cross-coupling reaction of organosilanes with organic halides to form carbon-carbon bonds. R X R' catalyst SiR'' 3 R R' This reaction has been applied to the synthesis of natural products. Example 6 OH Si O O I Br Si O H C H 3O 6 VUOS – PARTNER FOR COOPERATION C H 3O STILLE REACTION The reaction is a cross-coupling an organotin compound with an sp2-hybridized organic halide catalyzed by palladium. R X catalyst R' Sn(R'')3 R R' The reaction continues to be exploited industrially, especially for pharmaceuticals. Example O 7 O Cl S n(C 4 H 9 ) 3 O H 3C H 3C O Br C H 3O O C 7 C H3 S n(C 4 H 9 ) 3 VUOS – PARTNER FOR COOPERATION C H 3O O C C H3 HECK REACTION The reaction is a coupling of an unsaturated halide (or triflate) with an alkene and a base and palladium catalyst to form a substituted alkene. R' catalyst R X R' R This reaction is of great importance, as it allows one to do substitution reactions on planar centers. 8 Example I O OH I O 8 VUOS – PARTNER FOR COOPERATION OH SONOGASHIRA REACTION The reaction is a cross-coupling reaction using a palladium catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide. catalyst HC C R R' X R' C C R The Sonogashira cross-coupling reaction has been employed in a wide variety of areas. 9 Example O O OH O O I OH I I 9 VUOS – PARTNER FOR COOPERATION BUCHWALD–HARTWIG AMINATION The reaction is used for the synthesis of carbon–nitrogen bonds via the palladium-catalyzed cross-coupling of amines with aryl halides. R R' NH X catalyst R' R N R'' R'' The development of the reaction allowed for the facile synthesis of aryl amines, replacing to an extent harsher methods while significantly expanding the repertoire of possible C–N bond formation. 10 Example Br Cl C H 3N H C 2H 5 Br N O O H 2N Br (C 2 H 5 ) 2 N 10 VUOS – PARTNER FOR COOPERATION NH (C 2 H 5 ) 2 N FUKUYAMA REACTION The reaction is a coupling taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. O O catalyst R' Zn X R S R R'' R' Advantages are high chemoselectivity, mild reaction conditions and the use of less-toxic reagents. 11 Example O C F3 S S I Zn O C F3 O O S O 11 Zn Cl O VUOS – PARTNER FOR COOPERATION O O α-ARYLATION REACTION This coupling reaction between arylhalide and organic compound containing C-O group by using a palladium-complex catalyst. O O catalyst Ar X R' R' R R Ar Advantages are mild reaction conditions and high chemoselectivity. Example 12 C H3 C H3 C H3 C H3 Br O O O Br O C H3 O O H 3C 12 VUOS – PARTNER FOR COOPERATION INTRODUCTION OF CARBOXYLIC GROUP This reaction is a coupling between arylhalide and cabron dioxide. As catalyst can be used a palladium-complex catalyst activated with organometallic compound. catalyst R X CO2 R COOH This reaction allows to expand a posibilities of carboxylic group introducing. 13 Example F 3C C F3 F 3C C F3 CO2 Br COOH COOH Br CO2 O 13 O VUOS – PARTNER FOR COOPERATION 14 14