CMC Chapter 18
advertisement

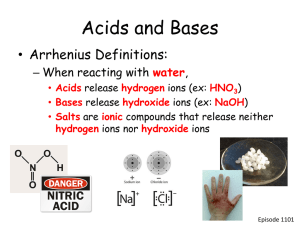
Acids and Bases Section 18.1 Introduction to Acids and Bases Section 18.2 Strengths of Acids and Bases Section 18.3 Hydrogen Ions and pH Section 18.4 Neutralization Click a hyperlink or folder tab to view the corresponding slides. Exit Section 18.1 Introduction to Acids and Bases • Identify the physical and chemical properties of acids and bases. • Classify solutions as acidic, basic, or neutral. • Compare the Arrhenius, Brønsted-Lowry, and Lewis models of acids and bases. Lewis structure: a model that uses electron-dot structures to show how electrons are arranged in molecules Section 18.1 Introduction to Acids and Bases (cont.) acidic solution conjugate base basic solution conjugate acid-base pair Arrhenius model amphoteric Brønsted-Lowry model Lewis model conjugate acid Different models help describe the behavior of acids and bases. Properties of Acids and Bases • Acids taste sour. Bases taste bitter and feel slippery. • Acids and bases are conductors of electricity. • Acids and bases can be identified by their reactions with some metals and metal carbonates. Properties of Acids and Bases (cont.) • Acids turn blue litmus red. • Bases turn red litmus blue. • Magnesium and zinc react with acids to produce hydrogen gas. • Geologists identify limestone because it produces bubbles of carbon dioxide when exposed to hydrochloric acid. Properties of Acids and Bases (cont.) • All water solutions contain hydrogen ions (H+) and hydroxide ions (OH–). • An acidic solution contains more hydrogen ions than hydroxide ions. • A basic solution contains more hydroxide ions than hydrogen ions. Properties of Acids and Bases (cont.) • The usual solvent for acids and bases is water—water produces equal numbers of hydrogen and hydroxide ions in a process called self-ionization. H2O(l) + H2O(l) ↔ H3O+(aq) + OH–(aq) • The hydronium ion is H3O+. The Arrhenius Model • The Arrhenius model states that an acid is a substance that contains hydrogen and ionizes to produce hydrogen ions in aqueous solution, and a base is a substance that contains a hydroxide group and dissociates to produce a hydroxide ion in solution. The Arrhenius Model (cont.) • Arrhenius acids and bases – HCl ionizes to produce H+ ions. – HCl(g) → H+(aq) + Cl–(aq) – NaOH dissociates to produce OH– ions. – NaOH(s) → Na+(aq) + OH–(aq) – Some solutions produce hydroxide ions even though they do not contain a hydroxide group. The Brønsted-Lowry Model • The Brønsted-Lowry Model of acids and bases states that an acid is a hydrogen ion donor, and a base is a hydrogen ion acceptor. • The Brønsted-Lowry Model is a more inclusive model of acids and bases. The Brønsted-Lowry Model (cont.) • A conjugate acid is the species produced when a base accepts a hydrogen ion. • A conjugate base is the species produced when an acid donates a hydrogen ion. • A conjugate acid-base pair consists of two substances related to each other by donating and accepting a single hydrogen ion. The Brønsted-Lowry Model (cont.) • Hydrogen fluoride—a Brønsted-Lowry acid – HF(aq) + H2O(l) ↔ H3O+(aq) + F–(aq) – HF = acid, H2O = base, H3O+ = conjugate acid, F– = conjugate base The Brønsted-Lowry Model (cont.) • Ammonia— Brønsted-Lowry base – NH3(aq) + H2O(l) ↔ NH4+(aq) + OH–(aq) – NH3 = base, H2O(l) = acid, NH4+ = conjugate acid, OH– = conjugate base • Water and other substances that can act as acids or bases are called amphoteric. Monoprotic and Polyprotic Acids • An acid that can donate only one hydrogen ion is a monoprotic acid. • Only ionizable hydrogen atoms can be donated. Monoprotic and Polyprotic Acids (cont.) • Acids that can donate more than one hydrogen ion are polyprotic acids. The Lewis Model • According to the Lewis model, a Lewis acid is an electron-pair acceptor and a Lewis base is an electron pair donor. • The Lewis model includes all the substances classified as Brønsted-Lowry acids and bases and many more. Section 18.1 Assessment A Lewis acid is a(n) ____. A. electron pair donor B. hydrogen ion donor C. electron pair acceptor D C A 0% B D. substance that contains an hydroxide group A. A B. B C. C 0% 0% 0% D. D Section 18.1 Assessment A conjugate acid is formed when: A. a base accepts a hydrogen ion B. an acid accepts a hydrogen ion C. an acid donates a hydrogen ion D C A 0% B D. a base donates a hydrogen ion A. A B. B C. C 0% 0% 0% D. D Section 18.2 Strengths of Acids and Bases • Relate the strength of an acid or base to its degree of ionization. • Compare the strength of a weak acid with the strength of its conjugate base. • Explain the relationship between the strengths of acids and bases and the values of their ionization constants. electrolyte: an ionic compound whose aqueous solution conducts an electric current Section 18.2 Strengths of Acids and Bases (cont.) strong acid weak acid acid ionization constant strong base weak base base ionization constant In solution, strong acids and bases ionize completely, but weak acids and bases ionize only partially. Strengths of Acids • Acids that ionize completely are strong acids. • Because they produce the maximum number of hydrogen ions, strong acids are good conductors of electricity. Strengths of Acids (cont.) • Acids that ionize only partially in dilute aqueous solutions are called weak acids. Strengths of Acids (cont.) • With a strong acid, the conjugate base is a weak base. • Equilibrium lies almost completely to the right in the equation because the conjugate base has a weaker attraction for the H+ ion than does the base in the forward reaction. • In a weak acid, the ionization equilibrium lies to the far left in the ionization equation because the conjugate base has a greater attraction for H+ ions than does the base in the forward reaction. Strengths of Acids (cont.) • The equilibrium constant, Keq, provides a quantitative measure of the degree of ionization of an acid. • The acid ionization constant is the value of the equilibrium constant expression for the ionization of a weak acid, Ka. • Ka indicates whether products or reactants are favored at equilibrium. Strengths of Acids (cont.) • For weak acids, the products tend to be smaller compared to the un-ionized molecules (reactant). • Weaker acids have a smaller Ka. Strengths of Bases • A base that dissociates completely into metal ions and hydroxide ions is known as a strong base. • A weak base ionizes only partially in dilute aqueous solution. Strengths of Bases (cont.) • The base ionization constant, Kb, is the value of the equilibrium constant expression for the ionization of a base. Section 18.2 Assessment A solution with a small Kb is a ____. A. weak acid B. weak base C. strong acid D C A 0% B D. strong base A. A B. B C. C 0% 0% 0% D. D Section 18.2 Assessment Where is the equilibrium point in the ionization equation for a strong acid? A. far right B. far left D A 0% C D. slightly left A. A B. B C. C 0% 0% 0% D. D B C. slightly right Section 18.3 Hydrogen Ions and pH • Explain pH and pOH. • Relate pH and pOH to the ion product constant for water. • Calculate the pH and pOH of aqueous solutions. Le Châtelier’s principle: states that if a stress is applied to a system at equilibrium, the system shifts in the direction that relieves the stress ion product constant pH and pOH are logarithmic for water scales that express the pH concentrations of hydrogen pOH ions and hydroxide ions in aqueous solutions. Ion Product Constant for Water • Pure water contains equal concentrations of H+ and OH– ions. • The ion production of water, Kw = [H+][OH–]. • The ion product constant for water is the value of the equilibrium constant expression for the self-ionization of water. Ion Product Constant for Water (cont.) • With pure water at 398 K, both [H+] and [OH–] are equal to 1.0 × 10–7M. Kw at 298 K = 1.0 × 10–14 • Kw and LeChâtelier’s Principle proves [H+] × [OH–] must equal 1.0 × 10–14 at 298 K, and as [H+] goes up, [OH–] must go down. pH and pOH • Concentrations of H+ ions are often small numbers expressed in exponential notation. • pH is the negative logarithm of the hydrogen ion concentration of a solution. pH = –log [H+] pH and pOH (cont.) • pOH of a solution is the negative logarithm of the hydroxide ion concentration. • pOH = –log [OH–] • The sum of pH and pOH equals 14. pH and pOH (cont.) • For all strong monoprotic acids, the concentration of the acid is the concentration of H+ ions. • For all strong bases, the concentration of the OH– ions available is the concentration of OH–. • Weak acids and weak bases only partially ionize and Ka and Kb values must be used. pH and pOH (cont.) • Litmus paper and a pH meter with electrodes can determine the pH of a solution. Section 18.3 Assessment In dilute aqueous solution, as [H+] increases: A. pH decreases B. pOH increases D A 0% C D. all of the above A. A B. B C. C 0% 0% 0% D. D B C. [OH–] decreases Section 18.3 Assessment What is the pH of a neutral solution such as pure water? A. 0 B. 7 D A 0% C D. 1.0 × 10–14 A. A B. B C. C 0% 0% 0% D. D B C. 14 Section 18.4 Neutralization • Write chemical equations for neutralization reactions. • Explain how neutralization reactions are used in acid-base titrations. • Compare the properties of buffered and unbuffered solutions. stoichiometry: the study of quantitative relationships between the amounts of reactants used and products formed by a chemical reaction; is based on the law of conservation of mass Section 18.4 Neutralization (cont.) neutralization reaction acid-base indicator salt end point titration salt hydrolysis titrant buffer equivalence point buffer capacity In a neutralization reaction, an acid reacts with a base to produce a salt and water. Reactions Between Acids and Bases • A neutralization reaction is a reaction in which an acid and a base in an aqueous solution react to produce a salt and water. • A salt is an ionic compound made up of a cation from a base and an anion from an acid. • Neutralization is a double-replacement reaction. Reactions Between Acids and Bases (cont.) Reactions Between Acids and Bases (cont.) • Titration is a method for determining the concentration of a solution by reacting a known volume of that solution with a solution of known concentration. Reactions Between Acids and Bases (cont.) • In a titration procedure, a measured volume of an acid or base of unknown concentration is placed in a beaker, and initial pH recorded. • A buret is filled with the titrating solution of known concentration, called a titrant. Reactions Between Acids and Bases (cont.) • Measured volumes of the standard solution are added slowly and mixed into the solution in the beaker, and the pH is read and recorded after each addition. The process continues until the reaction reaches the equivalence point, which is the point at which moles of H+ ion from the acid equals moles of OH– ion from the base. • An abrupt change in pH occurs at the equivalence point. Reactions Between Acids and Bases (cont.) Reactions Between Acids and Bases (cont.) • Chemical dyes whose color are affected by acidic and basic solutions are called acidbase indicators. Reactions Between Acids and Bases (cont.) • An end point is the point at which an indicator used in a titration changes color. • An indicator will change color at the equivalence point. Salt Hydrolysis • In salt hydrolysis, the anions of the dissociated salt accept hydrogen ions from water or the cations of the dissociated salt donate hydrogen ions to water. Salt Hydrolysis (cont.) • Salts that produce basic solutions − KF is the salt of a strong base (KOH) and a weak acid (HF). KF(s) → K+(aq) + F–(aq) Salt Hydrolysis (cont.) • Salts that produce acidic solutions − NH4Cl is the salt of a weak base (NH3) and strong acid (HCl). − When dissolved in water, the salt dissociates into ammonium ions and chloride ions. NH4Cl(s) → NH4+(aq) + Cl–(aq) Salt Hydrolysis (cont.) • Salts that produce neutral solutions − NaNO3 is the salt of a strong acid (HNO3) and a strong base (NaOH). − Little or no salt hydrolysis occurs because neither Na+ nor NO3– react with water. Buffered Solutions • The pH of blood must be kept in within a narrow range. • Buffers are solutions that resist changes in pH when limited amounts of acid or base are added. Buffered Solutions (cont.) • Ions and molecules in a buffer solution resist changes in pH by reacting with any hydrogen ions of hydroxide ions added to the buffered solution. HF(aq) ↔ H+(aq) + F–(aq) • When acid is added, the equilibrium shifts to the left. Buffered Solutions (cont.) • Additional H+ ions react with F– ions to form undissociated HF molecules but the pH changes little. • The amount of acid or base that a buffer solution can absorb without a significant change in pH is called the buffer capacity. Buffered Solutions (cont.) • A buffer is most effective when the concentrations of the conjugate acid-base pair are equal or nearly equal. Section 18.4 Assessment In a neutralization reaction, an acid and base react to form: A. salt and oxygen gas B. salt and ammonia D A 0% C D. precipitate and water A. A B. B C. C 0% 0% 0% D. D B C. salt and water Section 18.4 Assessment Solutions that resist changes in pH are called ____. A. titrants B. salts D A 0% C D. buffers A. A B. B C. C 0% 0% 0% D. D B C. conjugate pairs Chemistry Online Study Guide Chapter Assessment Standardized Test Practice Image Bank Concepts in Motion Section 18.1 Introduction to Acids and Bases Key Concepts • The concentrations of hydrogen ions and hydroxide ions determine whether an aqueous solution is acidic, basic, or neutral. • An Arrhenius acid must contain an ionizable hydrogen atom. An Arrhennius base must contain an ionizable hydroxide group. • A Brønsted-Lowry acid is a hydrogen ion donor. A Brønsted-Lowry base is a hydrogen ion acceptor. • A Lewis acid accepts an electron pair. A Lewis base donates an electron pair. Section 18.2 Strengths of Acids and Bases Key Concepts • Strong acids and strong bases are completely ionized in a dilute aqueous solution. Weak acids and weak bases are partially ionized in a dilute aqueous solution. • For weak acids and weak bases, the value of the acid or base ionization constant is a measure of the strength of the acid or base. Section 18.3 Hydrogen Ions and pH Key Concepts • The ion product constant for water, Kw, equals the product of the H+ ion concentration and the OH– ion concentration. Kw = [H+][OH–] • The pH of a solution is the negative log of the hydrogen ion concentration. The pOH is the negative log of the hydroxide ion concentration. pH plus pOH equals 14. pH = –log [H+] pOH = –log [OH–] pH + pOH = 14.00 • A neutral solution has a pH of 7.0 and a pOH of 7.0 because the concentrations of hydrogen ions and hydroxide ions are equal. Section 18.4 Neutralization Key Concepts • In a neutralization reaction, an acid and a base react to form a salt and water. • The net ionic equation for the neutralization of a strong acid by a strong base is H+(aq) + OH–(aq) → H2O(l). • Titration is the process in which an acid-base neutralization reaction is used to determine the concentration of a solution. • Buffered solutions contain mixtures of molecules and ions that resist changes in pH. Which type of acid accepts an electron pair? A. Arrhenius B. Brønsted-Lowry D A 0% C D. Dalton A. A B. B C. C 0% 0% 0% D. D B C. Lewis What is the conjugate of a weak acid? A. strong acid B. strong base C. weak acid D C A 0% B D. weak base A. A B. B C. C 0% 0% 0% D. D In a solution with a pH of 4.0, which of the following is true? A. [H+] > [OH–] B. [H+] < [OH–] D A 0% C D. none of the above A. A B. B C. C 0% 0% 0% D. D B C. [H+] = [OH–] Salt hydrolysis is what type of reaction? A. single-replacement B. double replacement C. synthesis D C A 0% B D. decomposition A. A B. B C. C 0% 0% 0% D. D The strength of a weak acid is measured by: A. ion product constant B. base ionization constant D A 0% C D. acid ionization constant A. A B. B C. C 0% 0% 0% D. D B C. pOH What is the pOH of 0.250M HBr, a strong acid? A. 0.60 B. 0.80 D A 0% C D. 13.2 A. A B. B C. C 0% 0% 0% D. D B C. 13.4 Which of the following is a diprotic acid? A. CH2COOH B. HCl D A 0% C D. H2SO4 A. A B. B C. C 0% 0% 0% D. D B C. NH3+ What is the conjugate acid in the following equation? HCl(s) + H2O → H3O+(aq) + Cl–(aq) A. Cl– A 0% D D. HCl C C. H2O A. A B. B C. C 0% 0% 0% D. D B B. H3O+ An increase in concentration of hydrogen ions when hydroxide ions are added to an aqueous solution is an example of: A. an Arrhenius acid A 0% D D. Le Châtelier’s Principle C C. the equivalence point A. A B. B C. C 0% 0% 0% D. D B B. a Brønsted-Lowry acid Which of the following is a polar molecule? A. CH4 B. PCl5 D A 0% C D. H2 A. A B. B C. C 0% 0% 0% D. D B C. H2O Click on an image to enlarge. Table 18.2 Three Models for Acids and Bases Table 18.3 Ionization Equations Figure 18.20 A Neutralization Reaction Figure 18.21 Titration Figure 18.22 Neutralization Reactions Click any of the background top tabs to display the respective folder. Within the Chapter Outline, clicking a section tab on the right side of the screen will bring you to the first slide in each respective section. Simple navigation buttons will allow you to progress to the next slide or the previous slide. The Chapter Resources Menu will allow you to access chapter specific resources from the Chapter Menu or any Chapter Outline slide. From within any feature, click the Resources tab to return to this slide. The “Return” button will allow you to return to the slide that you were viewing when you clicked either the Resources or Help tab. To exit the presentation, click the Exit button on the Chapter Menu slide or hit Escape [Esc] on your keyboards while viewing any Chapter Outline slide. This slide is intentionally blank.