Chapter 13
advertisement

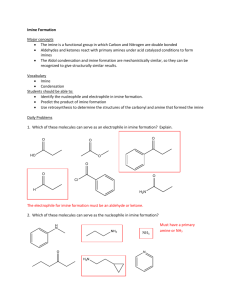
for the first time: carbon nucleophiles, leaving groups breaking / forming carbon-carbon binds E O O: C carbanion nucleophile R C CR 3 R R C + :CR3 R carbanion leaving group intro carbon Nu, LG in biological reactions are usually enolates: O R O CR2 R O: CR2 H R CR2 enolate ion :B sometimes, carbon nucleophiles are from alkenes (electrophilic addition) E C C Keto-enol tautomerization tautomers: different consitutional isomers in rapid equilibrium O O H3C C H3C CH3 keto form of acetone R R H keto B O O: R R CH2 enol form of acetone H O C H :B R R enolate H R R R enol 13.1A usually, keto form predominates, but there are exceptions: H O O keto form (24%) O O enol form (76%) 13.1A imine – enamine tautomerization N H N: C C C H C enamine imine eg: degration of serine: NH2 O H A NH2 O CH3 CH2 O enamine O imine H2O NH3 O O CH3 O pyruvate 13.1B Isomerization reactions carbonyl isomerization: triose-phosphate isomerase H A :B O OH PO OH HS PO 3 2 HR O 1 H H :B DHAP (ketone) HO H H A ene-diol PO O H GAP (aldehyde) 13.2A OH OP OH OH O OH glucose-6-phosphate OH OP OH OH OH O fructose-6-phosphate 13.2A stereoisomerization: racemization OH PO O 4 (R) 2 3 5 OH OH PO HO H xylulose-5-phosphate ribulose-5-phosphate Zn PO OH (S) 1 HO H OH O Zn O OH OH 3 Zn OH O: PO OH O PO OH 1 6 HO H HO H OH O H O Asp1 O O Asp2 13.2B The aldol reaction (carbon-carbon bond forming) what happens to an enolate? It can go to the enol (oxygen acts as base): H A OH O: R R R R R R enolate enol 13.3A it can go back to the keto (carbon acts as base): O: O H A R R R R R R enolate O R R H R keto . . .or carbon can act as a nucleophile O O O R C R R R R C R C H A OH R R C R O R R C R O = R R R OH C C R R R on the electrophilic side of the picture, this is just a carbonyl addition! 13.3A the ‘classic’ aldol reaction: self-condensation of an aldehyde A H O H O H R H R H aldehyde R R H O: C C C R alcohol O R H H OH C R C R R H R :B 13.3A best-studied biochemical aldol reaction: fructose 1,6bisphosphate aldolase new C-C bond OH OH OH OP OH OP + OP GAP O O DHAP OP OH O fructose-1,6-bisphosphate 13.3B ‘class II’ aldolase: enolate stabilized by Zn2+ H OH OH OP OP H H B: O O: Zn Zn new stereocenter OH OP OH OH OP H OH O: H Zn OP OH O Zn OP O H A 13.3B ‘Class I aldolase: enamine intermediate rather than enolate first, Schiff base (imine) forms with enzyme lysine: OH OP H2O O NH3 enz OH OP N enz 13.3B H OH OH OP H H B: OP H A N enz NH enz enamine 13.3B OH OP OH OH OP H2O OH OH OP NH H OP OH OP OH OP NH OH O NH3 enz O enz enz H A 13.3B eg: methanotrophs incorporating (toxic) formaldehyde: OH O PO OH O PO OH + H OH H HO H HO H ribulose-5-phosphate O formaldehyde OH hexulose-6-phosphate draw the key enamine intermediate! 13.3B thioesters, esters can also be the nucleophilic half of an aldol reaction: :A H A B: H O H C O2C O SCoA H2C H H O2C SCoA SCoA O OH O H A CO2 CO2 (but electrophilic half must be aldehyde-ketone – if it’s a thioester or ester, something else happens! 13.3B aldol reactions are reversable: retro-aldol fructose 1,6,-bisphosphate in reverse (sugar-breaking) direction: OH OH OP OH OH OP + OP OH O OP fructose-1,6-bisphosphate OH OH O DHAP GAP OH OP O H A OP OH OH OP + OP O O H Zn OP O O: O Zn B: 13.3C recognizing a potential retro-aldol: look for alcohol b to carbonyl (or imine) :B O R H ??? R no retro-aldol possible! Leaving group is unstabilized carbanion. O X + R R 13.3C here, OH is b to imine: :B H H A N H (enamine) ( t r y p t OH OP OH O H tautomerization OP OH N H (imine) H O OP + N H OH 13.2C transaldol reactions: retro followed by forward OH OH O O HO O + OH OH OH OH OP OP fructose-6phosphate erythrose-4phosphate HO O OH + OP glyceraldehyde3-phosphate OH OH OH OP sedoheptulose-7phosphate 13.3D imine link to active site lysine OH N OH N Lys H A HO Lys H HO retroaldol O H OH :B OP fructose-6-phosphate O OH OP glyceraldehyde3-phosphate released from active site OH N HO Lys N H aldol O enters active site OH OH H OH OH OP erythrose4-phosphate A HO H O Lys H2O HO OH OH OH OH OH OH OP OP sedoheptulose-7phosphate 13.3D Claisen reaction enolate nucleophile, carboxylic acid derivative as electrophile O O: R R O X R R O: X O R O R new carboncarbon bond from the electrophile side, this is an acyl transfer reaction! 13.4 eg. early cholesterol biosynthesis: condensation of two acetyl CoA units O HSCoA O SCoA O: O Cys 1 SCoA S SH SCoA 2 H B: Cys 3 O: O O SCoA S 4 O SCoA Cys 13.4A Claisens can also go backward (retro-Claisen) key to recognition: look for b-keto (thio)ester! eg. fatty acid degradation: first, b-keto group is introduced: O R b O 3 steps SCoA R b O SCoA fatty acyl CoA (we’ll see these reactions later) 13.4B O O R SCoA H B: O R O O SCoA S R O: S SCoA Cys Cys H A S HSCoA Cys fatty acid has lost two carbons O O R SCoA SCoA acetyl CoA . . .just an acyl substitution reaction with a carbanion (enolate) LG! 13.4B another example: (degradation of Tyr/Phe) :B H O H H O2C CO2 O O O2C CO2 O O Mg2+ O Mg 2+ O2C O CO2 + O O 13.4B occasionally, enol/enolate can attack in SN2 fashion: H :B O O H3C CO2 H N H CO2 R C S H H SAM R N H in lab synthesis, alkyl halide (eg. CH3I) is used – we’ll see this soon 13.4C Carboxylation: aldol, with CO2 as electrophile: Rubisco (carbon fixation by plants, some bacteria): Mg OH PO O 4 OP 2 1 5 HO H OH step 1 :B ribulose-1,5-bisphosphate Mg O PO OH OP step 2 OH PO O OP O O C H O step 3 OP OH PO OH CO2 O 13.5B . . .second part of Rubisco reaction: OP OH H2O OH PO OH OH PO O O + PO O step 4 O O O O phosphoglycerate notice: retro-Claisen! 13.5B Decarboxylation O b carbonyl group O O O + R O R C CH2 H O A O R OH R O X + R CH2 O O OH O CH3 C R O O O no decarboxylation! CO2 is NOT the leaving group! 13.5C examples: O O CO2 O2C O2C CO2 CO2 CO2 CO2 OH OH CO2 O O OH OH OH OH OP OP 13.5C decarboxylation of imine: Lys Lys H2O O CO2 N CO2 N H2O O CO2 13.5C variation: decarboxylation (to form enolate), followed by Claisen O S ACP CO2 O O O O S ACP (ACP = acyl carrier protein) :O S ACP S O ACP S O ACP O S ACP (fatty acid biosynthesis) 13.5C what’s happening here? CO2 CO2 CO2 CO2 O O HO O (biosynthesis of Tyrosine) 13.5C Carbanion reactions the lab acetoacetic acid synthesis: O O O 1) NaOCH2CH3 3) NaOH (aq) 2) R Br 4) HCl (aq) O R NaBr + CO2 2 EtOH malonic ester synthesis: O EtO O O OEt 1) NaOCH2CH3 3) NaOH (aq) 2) R Br 4) HCl (aq) HO NaBr R + CO2 3 EtOH you get to write out the mechanisms! 13.6A skip to section 13.6C more carbon nucleophiles in the lab another way to make a carbon nucleophile: deprotonate a terminal alkyne: pKa ~ 19 Na R C C H Na+ NH2:NH3 (l) R R C C CH2R C C: R Na Br H C Br H then, can make cis / trans alkenes: R H C C R H CH2R R C C CH2R H C C CH2R H 13.6C The Grignard reaction H C Br + Mg(0) R H C O R2 C O R1 R3 MgBr H H H C MgBr + reacts like R R C MgBr H R1 H H C R2 H+ R3 R1 OH C R2 (plus enantiomer) R3 13.6B OH O Br MgBr H3C + Mg(0) C CH3 H+ C CH3 CH3 O MgBr CO2 H+ C OH 13.6D Grignard plus epoxide: 1) MgBr OH O CH3 + 2) H3O+ CH3 Grignards react twice with esters, acid chloride O H3C C O MgBr O CH3 +2 H3C C + CH3O OH H3C C O H3O+ H 3C C question: what about Grignard plus carboxylic acid? 13.6B