Chemical Properties of Seawater
advertisement

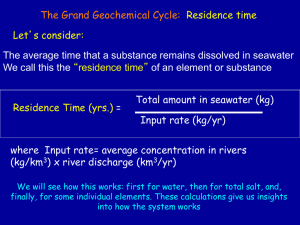
Learning Objectives Understand water’s structure and unique properties Define solutions and their properties Determine what properties change when a solute is added to a solvent Define and explain colligative properties and interactions of a solution Describe seawater’s properties and chemical composition Discuss the environmental issues associated with seawater’s chemical properties. Water’s Chemical Structure Chemical Structure: 1- Oxygen and Hydrogen bonds-hydrogen shares one pair of electron with oxygen. 2- Two unpaired electron pairs are unbonded 3- Electrons shared with oxygen are strongly attracted towards the oxygen atom. (this is called electronegativity) 4- The unequal sharing of electrons create a charge difference. Hydrogen has slight positive charge and oxygen has a slight negative charge. 5- The structure formed is a bent polar molecular structure. Also called a Dipole molecule. 105° Angle, 2 bonded pairs and 2 unshared pairs. H2O Lab 1- Draw a Bohr’s model for each of elements listed below: Hydrogen, Oxygen, Sodium, Chloride, Calcium, Magnesium, Potassium, Nitrogen, Phosphorus, Carbon and Fluoride. 2- Using the molecular model kits build a model of water and other chemical compounds. Include these compounds: Carbon dioxide Sodium Chloride Bicarbonate Use the following website to help you: http://www.stolaf.edu/depts/chemistry/mo/struc/explore. htm Water Properties Due to the hydrogen bonds in water, water’s freezing and boiling points are much higher than other substances. Water has a high heat capacity, which means it takes a great deal of energy to change the state of water. This is an important factor when discussing ocean currents and atmospheric conditions. Heat Capacity of Water Discussion: • Which heats up faster, water or land? Give an example of this thermal interaction. Watch the video from How Stuff Works • http://videos.howstuffworks.com/hsw/8451-waterheat-capacity-video.htm Properties of Solutions Solution- a homogenous mixture of two or more substances in a single physical state. very small evenly distributed-uniformly will not separate no matter how long it is allowed to stand Solute-substance that is dissolved Solvent-substance that does the dissolving Example: Salt Water- Salt-Solute, Water-Solvent Properties of Solutions Soluble- a substance that dissolves in another substance. Example: Salt and Water Insoluble-a substance that does not dissolve in another Example: Oil and Water Polar solutes tend to dissolve in polar solvents. Example: Water is the universal solvent due to its polar molecule. Salt Water. Grease or oil do not dissolve in water because both are nonpolar molecule. (Insoluble) Ionic compounds do dissolve in water because they have charges which make them polar compounds Demonstration: Cooking Oil and Water Free-StockPhotos.com Time Warp: Oil in Water http://dsc.discovery.co m/videos/time-warpoil-and-water.html Ocean Connection Problem: Oil Spills and Oil Waste • Using the information on solutions and the website below, explain why oil spills and waste are an environmental problem for oceans. http://www.waterencyclopedia.com/OcPo/Oil-Spills-Impact-on-the-Ocean.html Photo from website Temperature and Pressure solutions of gases in liquids are greatly affected by changes in temperature: Soda As temperature increases the solubility of a gas in a liquid decreases. The effect of temperature changes on the solubility of solids in liquids is very different from that of gases. Solubility of solid solute increases, as temperature increases. solubility of a gas in a liquid is strongly influenced by pressure The solubility of a gas in a solvent is increased, when the pressure is increased Lab 1-Gas Simulation Lab Learning goal: To understand the properties and behavior of gases under certain conditions, particularly under changing temperature, pressure and volume. Use the website below to answer all questions: http://phet.colorado.edu/simulations/sims.php?sim=Gas_Properties 2- Complete the Investigating How Temperature Affects Gas Solubility Lab Colligative Properties and Interactions Depends on the concentration of solute particles and their chemical identity Includes vapor pressure reduction, boiling point elevation, freezing point depression and osmotic pressure. Colligative interactions-surface tension, viscosity, cohesion and density Seawater Properties Seawater is considered a solution Water dissolves salt (NaCl) an ionic compound by breaking the bonds of between ions . Salinity: the total amount of dissolved salt. Units: parts per thousands (ppt) Example: Seawater 35 ppt. which translates to 0.26 gallons (1kg) of seawater contains about 1.13 ounces (35 g) of dissolved salts Click for Animation coolcosmos.ipac.caltech.edu/.../tempscales.html Physical Properties Seawater demonstrates colligative properties: Salt (solute) lowers the freezing point of water and raises the boiling the pt of water. The freezing and boiling point will depend on the salinity of the seawater. An example of boiling point elevation can be seen near the hydrothermal vents at the midocean ridges. Density- is controlled by salinity, pressure and temperature. Greater than pure water because of dissolved salts. Also depends on temperature- example cooling surface water with less salt content will increase in density and sink. http://coolcosmos.ipac.caltech.ed u/cosmic_classroom/cosmic_refer ence/tempscales.html Colligative Interactions Salt added to water increases the surface tension Salt added to water increases the viscosity of water, however by a small amount. Surface water at the equator is warmer, decreasing the viscosity of seawater. Temperature effects both interactions Click to the photo to view Teacher Tube Video Lab: Students should be able to understand the differences between water and seawater: (Lab is from “Life on an Ocean Planet: Activity #1 Chapter 6. Teachers can substitute a Lab of their choice) Complete “Water, More than just Wet, it’s unique” Lab Start with Station 2 “Study of Cohesion” Make sure you repeating the steps for each station with salt water solution (prepared by teacher) Complete all diagrams and label all information Conclusion: Explain the differences between salt water and pure water? Composition Trace elements are present in small concentration-parts per billion Major constituents are listed in the table and appear in seawater in minute quantities. The ionic composition of open-ocean water remains the same. A constant proportion is maintained (Marcet and Forchhammer). Give the chemical symbol, atomic mass, atomic number and what group they appear in on the periodic table. Table from An introduction to the World’s Oceans Sources of Salt Chemical weathering of rocks on the continents Earth’s interior- volcanic eruptions- water vapor and other gases-outgassing Salinity remains constant through time NOAA Website Gases Most gases are obtained from the atmosphere and distributed through depths by mixing processes. Nitrogen, Oxygen and Carbon Dioxide are the most abundant dissolved gases. Information taken from Introduction to the World’s Oceans Dissolved Gases Oxygen and Carbon Dioxide play important roles in the ocean for biological activities. Concentrations of Oxygen are high on surface waters, while Carbon Dioxide concentrations are low. As depth increases, oxygen levels decrease and carbon dioxide increases. Both are influenced by the biology. Photosynthesis takes place at the surface, as depth increases respiration increases and oxygen decreases. Carbon dioxide is added to deep waters, these deep waters can hold high concentrations of CO2 due to low temperatures and high pressure. Carbon dioxide in seawater reacts with water to form carbonic acid (H2CO3) Seawater pH Water is amphoteric-it can act as an acid or base As an Acid water gives up H+ to become OH As a base water accepts an H+ to become an H3O+ In pure water- H3O+ and OH- ions are found at a concentration of 1.0 x 10-7 M- pH 7- neutral Acidity or alkalinity of solutions are measured using the pH scale. Seawater is slightly alkaline with a pH between 7.5 and 8.5. Buffering-is a substance that prevents sudden changes in acidity or alkalinity of a solution. Carbon dioxide acts as a buffer, controlling the pH of seawater. Click the pH scale to view a parcel of water as its pH changes from acidic to alkaline Carbon dioxide and Carbonic Acid Chemistry CO2 + H2O H2CO3 HCO3 - + H+ or CO32- + 2H+ CO2 combines with the water form carbonic acid. Carbonic acid dissociates into bicarbonate, hydrogen ion and 2 hydrogen ions. •This helps to maintain a constant pH •pH of seawater depends on the concentration of CO2 •Higher concentrations of CO2more acidic seawater becomes •Warm water at the surface has a high pH 8.5 •Cold deep water is more acidic due to high concentrations of CO2 Environmental Concern Oxygen-deprived zones- - caused by sluggish circulation and oxygen-poor waters can reduce oxygen concentrations at intermediate depths. - these can occur from natural occurrences, such as cold water rising Click for Flash Animation from depths bringing nutrients especially nitrogen to the surface. Plankton and nekton growth occurs, when these organisms die, bacteria takes over and deplete the water of oxygen. - Currently fertilizer runoff from farms and lawns is fueling oxygen-deprived zones. - Climate change can also increase the occurrences of oxygen-deprived zones. - These zones do not sustain fish and will cause ecological and economic problems. Environmental Concern Ocean Acidity- one-third of carbon dioxide released by burning fossil fuels end up in the oceans - evidence shows the ocean’s natural ability to process carbon dioxide is being overwhelmed -since the industrial revolution there has been a 30% surge in acidity -continued emission of CO2 indicate ocean chemistry will change drastically, this hasn’t happened for million of years. -this will threaten a variety of calcite-secreting organisms. Image from: http://ww w.atmos.u md.edu/~ rjs/oco/ Sources "Chemical detectives follow nitrogen's elusive and essential trail in the ocean: the 'isotope effect' offers a new way to track nitrogen." Oceanus, Dec 2008 v47 i1 p33(2). Science Resource Center. Gale. 13 July 2009 <http://galenet.galegroup.com/servlet/SciRC?ste=1&docNum=A192258 578> "Ocean acidification: the biggest threat to our oceans?(Washington Watch)." BioScience, Nov 2007 v57 i10 p822(1). Science Resource Center. Gale. 13 July 2009 <http://galenet.galegroup.com/servlet/SciRC?ste=1&docNum=A171887 003> "Deep-ocean life where oxygen is scarce: oxygen-deprived zones are common and might become more so with climate change. Here life hangs on, with some unusual adaptations." American Scientist, SeptOct 2002 v90 i5 p436(9). Science Resource Center. Gale. 13 July 2009 <http://galenet.galegroup.com/servlet/SciRC?ste=1&docNum=A90570 698> Sources LeMay, B. R. (2002). Chemistry "Connections to Our Changing World". Upper Saddle River, New Jersey: Prentice Hall. Sverdrup, A. &. (2008). An Introduction to the World's Oceans. New York, New York: McGraw Hill. Lutgens, T. &. (2009). Earth Science. Upper Saddle River, New Jersey: Prentice Hall. NOAA. (2008, March 25). Retrieved July 13, 2009, from Monitoring Estuaries: http://oceanservice.noaa.gov/education/kits/estuaries/estuaries 10_monitoring.html Maryland, U. o. (2009, March 1). Orbiting Carbon Observatory. Retrieved July 13, 2009, from Orbiting Carbon Observatory: http://www.atmos.umd.edu/~rjs/oco/