Day_28 - Rose
advertisement

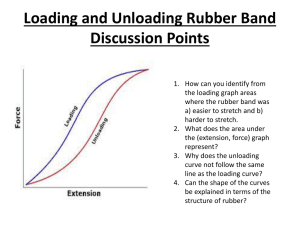
DAY 28: ELASTOMERS AND THERMOSETS What’s required for elastomeric behavior Essentials of elastomeric behavior Examples of Elastomers Example of a Thermoset THERMOSETS AND ELASTOMERS Both have some primary bonds between the chains. Because of the primary bonds, neither can be reground and reused. Elastomers show significant ELASTIC deformation. THE ISOPRENE MER Here is an important, naturally occuring mer. It exhibits what the text calls a “geometrical isomerism.” The double bond on the backbone is quite rigid and does not allow rotation from one form to the other. One of these mers will result in a natural “curlique” to the chain. Do you see which? THE CURL MAKES THE DIFFERENCE Polyisoprene “cis” has naturally occuring very coiled chains. It is the starting point for natural rubber. Polyisoprene “trans” has straighter chains. It is a material called gutta percha. Both are found in the sap of trees in Brazil and S.E. Asia. Interesting uses of gutta percha. 1. One of the earliest insulators. 2. “The guttie” core golf ball revolutionized golf. 3. Also used in filling tooth cores in dental work. 4. It is not highly elastic. MAKING RUBBER So far we have really coiled chains that might uncoil when loaded. They also might begin to slide past each other, ie. deform plastically. As the Macrogalleria notes, extending a piece of rubber decreases its entropy. MORE ON RUBBER To delay the onset of plastic deformation, a certain amount of crosslinking is created. One way of doing this is by adding sulfur. (Goodyear invented this process known as vulcanization.) What we have so far is: 1. Highly coilable chains. 2. Backbone bonds allow free rotation. 3. Crosslinking to prevent plastic deformation. Chains are fixed together by primary bonds at fixed locations. 4. Be above the glass temperature. THEORY OF RUBBER ELASTICITY Conventional elasticity. (I.e. metal spring) elastic strain energy stored in the primary bonds during deformation. Rubber elasticity. As we stretch, energy stored thermally. This is associated with an entropy decrease. (Heat transferred out of the rubber. Feels hot) There is then a thermodynamic “force” which drives us back towards high entropy (the original state.) So there is an entropy increase as we expand. (Heat transferred back into the rubber. Feels cool) ENTROPY SPRING Just as the conventional spring stores and releases energy, the rubber “entropy spring” rejects and accepts entropy. Further consequences: 1. Nonlinear stress strain behavior 2. Extremely large strains possible 3. Hysteris loop in the curve on unloading STRESS STRAIN CURVES Notes 1. Cycle dependence. First loading cycle results in permanent deformation. 2. 20th cycle curve is more typical. 3. Note large strains. The question of how to define strain becomes important. STRESS STRAIN CALCULATIONS IN RUBBER Please note: these materials are elastic, they are NOT linearly elastic. They fall into a very large materials class: the hyperelastic materials. In such materials, the stress is derived (conceptually) as follows: Theoretically and experimentally, we find a strain energy density function, V. Then, derive the following non-linear relationship for stress V ij ij SOMETHING TO REMEMBER For all practical purposes, rubber is brittle. There is no ductility. This is because of the crosslinking. This is confusing, because when we see large deformations in metals, we know there is ductility / plasticity at work. Not true in elastomers. Fracture surfaces show no macroscopic deformation. OTHER ELASTOMERS During WWII when access to the rubber trees was less, polymer chemists came up with ways to synthesize elastomers. Here is one, commonly used – polybutadiene. Again we see the double bond on the backbone of the polymer and the cis configuration. It will be coily. Widely used in automotive: tire treads, belts, hoses, gaskets, etc. SBS RUBBER – A THERMOPLASTIC ELASTOMER We start with what is called a “block copolymer.” By clever chemistry, we can get Like HIPS, but this one is a block, not a graft. In this case what happens is: Can be recycled!!! The polystyrene clumps together. These clumps do the job of crosslinks. SOME USES OF SBS ABS (ACRILONITRILE – CO –BUTADIENECO- STYRENE Here is the mer for PAN, polyacrilonitrile. Important source of fiber. Copolymerize it with styrene in what’s called an alternating copolymer There’s more! ABS We then copolymerize this with butadiene. Get ABS, a very strong, tough plastic. ABS PROPERTIES Material Density (g/cc) UTS (ksi) Ductility %EL Izod Impact Ft-lb/in ABS 1.07 5.8 50 2.45 Polycarbo 1.24 nate 8.2 14 2.5 It’s very light and tough. THERMOSETTING POLYMERS -- EPOXY We have to chemically make the polymer and form it all at the same time. Ingredient #2 Ingredient #1 EPOXY CHEMISTRY We get the two chemicals to react. The chemistry is complex. Curing, i.e. heating to promote the final reaction may be needed. The result is a 3D network solid. It will not soften and flow when heated. This is a thermoset. SOME PROPERTIES Note that Epoxy is quite strong and stiff. This is, of course, the unreinforced stuff. 1. 2. 3. 4. 5. Polymer Density g/cc UTS ksi %EL E ksi PVC 1.35 7.5 45 385 Epoxy 1.16 22.5 2 2500 Uses Electrical moldings, like some plugs Sinks Adhesives Coatings Matrix material for composites. http://www.ides.com/resinprice/resinpricingreport .asp POLYTETRAFLUOROETHANE (TEFLON) This is an interesting one. Here’s the mer It’s like PE but with the H’s replaced by fluorines. (F). This makes for a strange substance. 1. Secondary bonding is very strong between the highly polarized F’s. 2. The bonding is such that no other substances are attracted. This accounts for the no-stick. TEFLON PROPERTIES Very low coefficient of friction Dense No-stick Very good temperature resistance Kind of average strength good ductility Material Density g/cc UTS Ksi %EL E ksi HDPE 0.953 4.5 200 240 Teflon 2.16 5.3 400 --