Ch. 8 section 8.1 outline - Reeths
advertisement

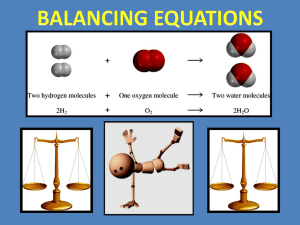
CHAPTER 8 CHEMICAL REACTIONS LEARNING THE PATTERNS OF CHEMICAL REACTIONS INTRODUCTION Section 8.1 Describing Chemical Change A. What is a CHEMICAL EQUATION? B. Why must an equation be balanced? INTRODUCTION Section 8.1 Describing Chemical Change A. What is a CHEMICAL EQUATION? • A chemical sentence that describes chemical changes. B. Why must an equation be balanced? • to obey the law of conservation of mass • mass of reactants must equal mass of products C. 5 TYPES OF CHEMICAL REACTIONS 1. Combination (synthesis) A + B AB 2 reactants (each just 1 element) 1 Product (a compound) (monatomic or diatomic) Example: 2H2 + O2 2H2O C. 5 TYPES OF CHEMICAL REACTIONS 1. Combination (synthesis) A + B AB 2. Decomposition AB A + B 1reactant (a compound) 2 Products (each just elements) C. 5 TYPES OF CHEMICAL REACTIONS 3. Single Replacement A + BC AC + B (A = metal) [metal, A, bonds with anion C] X + YZ YX + Z (X = nonmetal) [nonmetal, X, bonds with cation Y] C. 5 TYPES OF CHEMICAL REACTIONS 4. Double Replacement AB + CD AD + CB CATION FROM ONE COMPOUND REPLACES THE CATION FROM THE OTHERAND THE ANION FROM ONE WILL REPLACE THE ANION FROM THE OTHER. C. 5 TYPES OF CHEMICAL REACTIONS 5. COMBUSTION (burning a hydrocarbon) – CXHY + O2 XCO2 + y/2 H2O Combustion of Propane: C3H8 + 5 O2 3 CO2 + 4 H2O These are all examples of COMPLETE combustion C. 5 TYPES OF CHEMICAL REACTIONS 5. COMBUSTION, continued During COMPLETE combustion, there is enough oxygen to completely burn off all the fuel – the product is CO2. During INCOMPLETE combustion, CO is produced instead, since there is not enough oxygen available. CHEMICAL EQUATIONS D. Define REACTANTS - – starting substances E. Define PRODUCTS - – ending substances • Reactants products • “Reactants yield products” Table 8.1 Symbols Used in Equations Use your textbook, page 206, to write the symbols for the following words/phrases • • • • • • yields precipitate is made a gas is produced heat is being applied a catalyst is used a reversible reaction • • • • • solids liquids gases aqueous heat It would be in your best interest to commit these symbols and their meanings to memory. DEFINITIONS G. What is a skeleton equation? – not balanced yet H. What is a catalyst? – a substance that speeds up a reaction without being used up. Practice Problems • Write a skeleton equation for each of these chemical reactions 1. Aluminum metal reacts with oxygen in the air to form aluminum oxide. 2. When solid mercury (II) sulfide is heated with oxygen, liquid mercury metal and gaseous sulfur dioxide are produced 3. Oxygen gas can be made by heating potassium chlorate in the presence of the catalyst manganese dioxide. Potassium chloride is left as a solid residue.. Practice Problems • Write a skeleton equation for each of these chemical reactions 1. Aluminum metal reacts with oxygen in the air to form aluminum oxide. Practice Problems • Write a skeleton equation for each of these chemical reactions 1. Aluminum metal reacts with oxygen in the air to form aluminum oxide. Practice Problems • Write a skeleton equation for each of these chemical reactions 2. When solid mercury (II) sulfide is heated with oxygen, liquid mercury metal and gaseous sulfur dioxide are produced Practice Problems • Write a skeleton equation for each of these chemical reactions 2. When solid mercury (II) sulfide is heated with oxygen, liquid mercury metal and gaseous sulfur dioxide are produced Practice Problems • Write a skeleton equation for each of these chemical reactions 3. Oxygen gas can be made by heating potassium chlorate in the presence of the catalyst manganese dioxide. Potassium chloride is left as a solid residue.. Practice Problems • Write a skeleton equation for each of these chemical reactions 3. Oxygen gas can be made by heating potassium chlorate in the presence of the catalyst manganese dioxide. Potassium chloride is left as a solid residue.. • Write sentences that completely describe each of the chemical reactions shown in these skeleton equations. – KOH(aq) + H2SO4 (aq) H2O(l) + K2SO4(aq) – Na(s) + H2O(l) NaOH(aq) + H2(g) • Write sentences that completely describe each of the chemical reactions shown in these skeleton equations. – KOH(aq) + H2SO4 (aq) H2O(l) + K2SO4(aq) When potassium hydroxide, in aqueous solution combines with aqueous sulfuric acid, the result is liquid water and a potassium sulfate solution. – Na(s) + H2O(l) NaOH(aq) + H2(g) • Write sentences that completely describe each of the chemical reactions shown in these skeleton equations. – KOH(aq) + H2SO4 (aq) H2O(l) + K2SO4(aq) When potassium hydroxide, in aqueous solution combines with aqueous sulfuric acid, the result is liquid water and a potassium sulfate solution. – Na(s) + H2O(l) NaOH(aq) + H2(g) Solid sodium in liquid water yeilds a solution of sodium hydroxide and hydrogen bubbles. Balancing equations • Remember, in a chemical reaction, atoms are not created or destroyed, but simply rearranged. • In every balanced equation, each side of the equation has the same number of atoms of each element. Rules for Balancing Equations from pg. 208-209 • 1. Determine the correct formulas for all the reactants and products in the reaction. • 2. Write the formulas for the reactants on the left and the formulas for the products on the right with an arrow in between. If two or more reactants or products, separate them with a + sign • 3. Count the number of atoms of each element in the reactants and products. • 4. If a polyatomic ion appearing unchanged on both sides of the equation is counted as a single unit. Rules Con’t • 5. Balance the elements one at a time using coefficients. NEVER CHANGE A SUBSCRIPT TO BALANCE AN EQUATION!!!!! • CHECK EACH ATOM OR POLYATOMIC ION TO BE SURE THAT THE EQUATION IS BALANCED • 6. MAKE SURE COEFFICIENT RATIO IS LOWEST POSSIBLE RATIO. PRACTICE BALANCING • PROBLEM 3 PG 209 • PROBLEM 4 PG 209