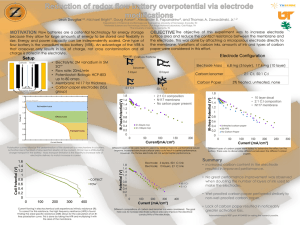
ion-selective electrode.
advertisement

14-6 Cells as chemical probes • It is important to distinguish two types of equilibrium in a galvanic cell: equilibrium between the two half-cells and equilibrium within each half cell. A galvanic cell that can be used to measure the formation constant of Hg(EDTA)2Hg2+ + 2e- ↔ Hg(l) Eo = 0.852 V 14-7 Biochemists use Eo’ • Formal potential: Potential of a half-reaction (relative to a standard hydrogen electrode) when the formal concentrations of reactants and products are unity. Any other conditions (such as pH, ionic strength, and concentrations of ligands) also must be specified. Finding the formal potential • Reduction potential of ascorbic acid, showing its dependence on pH. (a) Graph of the function labeled formal potential. (b) Experimental polarographic half-wave reduction potential of ascorbic acid in a medium of ionic strength = 0.2 M. At high pH (>12), the half-wave potential does not level off to a slope of 0, as Equation 14-34 predicts. Instead, a hydrolysis reaction of ascorbic acid occurs and the chemistry is more complex. • Example. Cadmium electrode immersed in a solution that is 0.015M in Cd2+ . Solution: Cd2+ + 2e Cd(s) 0 = -0.403 ε = εo - 0.0591 1 log 2 Cd 2+ 0.0591 1 ε = -0.403 log 0.457 V 2 0.0150 • Example: Calculate the potential for a platinum electrode immersed in a solution prepared by saturating a 0.015 M solution of KBr with Br2 Br2(l) + 2e 2BrE0 = 1.065 V Solution: 2 Br 0.0591 E= 1.065 log 2 1.0 E = 1.065 - 0.0591 2 log 0.0150 1.173V 2 Important notes 1. When you multiply a reaction by a coefficient, the potential of such a reaction does not change! 2. When you subtract or add reactions, the Gibbs energy of those reactions can always be directly added or subtracted, not their potentials! Chapter 15: Electrodes and potentiometry A few definition • Potentiometry: the use of electrodes to measure voltages that provide chemical information. • Electroactive species: a substance that can accept or donate electrons at an electrode. • Indicator electrode: one that develops a potential whose magnitude depends on the activity of one or more species in contact with the electrode. • Reference electrode: one that maintains constant potential against which the potential of another half-cell may be measured. 15-1 Reference electrodes • A galvanic cell that can monitor the quotient [Fe2+]/[Fe3+] in the right half-cell. The electrode potential E+ = 0.771 – 0.05916 log([Fe2+]/[Fe3+]) E- = 0.222 – 0.05916 log([Cl-]) E = E + - E- • The Pt wire is the indicator electrode. • Silver-silver chloride electrode: a reference electrode. Reference electrodes Silver-Silver Chloride Reference Electrode Calomel electrode • Figure shows a double-junction electrode that minimizes contact between analyte solution and KCl from the electrode. • A problem with reference electrodes is that porous plugs become clogged, thus causing sluggish, unstable electrical response. Some designs incorporate a free-flowing capillary in place of the porous plug. Potential for the solution saturated with KCl (is not 0.222 V): AgCl(s) + e- ↔ Ag(s) + ClEo = -0.197 V • A Hg electrode saturated with KCl is called a saturated calomel electrode, abbreviated S.C.E. The advantage in using saturated KCl is that [Cl−] does not change if some liquid evaporates. Ecalomel saturated with KCl 0.241 V Voltage conversions between different reference scales • If an electrode has a potential of −0.461 V with respect to a calomel electrode, what is the potential with respect to a silver-silver chloride electrode? What would be the potential with respect to the standard hydrogen electrode? 15-2 Indicator electrode • Metal electrodes: such as Pt, Au, Ag, etc. • Ion-selective electrodes: • Carbon electrode Example: Use of Ag and calomel electrodes to measure [Ag+]. 1 0.241 E 0.799 0.0591 log Ag Why do we need double junction calomel reference electrode here? 15-3 What is a junction potential • Whenever dissimilar electrolyte solutions are in contact, a voltage difference called the junction potential develops at their interface. This small voltage (usually a few millivolts) is found at each end of a salt bridge connecting two half-cells. The junction potential puts a fundamental limitation on the accuracy of direct potentiometric measurements. • The steady-state junction potential represents a balance between the unequal mobilities. Development of the junction potential caused by unequal mobilities of Na+ and Cl−. • Junction potential: An electric potential that exists at the junction between two different electrolyte solutions or substances. It arises in solutions as a result of unequal rates of diffusion of different ions. • A 0.1 M NaCl solution was placed in contact with a 0.1 M NaNO3. Which side of the junction is positive? 15-4 How ion-selective electrodes work • Respond selectively to one ion. • Do not involve redox processes. • Features a thin membrane capable of binding only the intended ion. • The electric potential difference across the ion-selective membrane is measured with two reference electrodes. 15-5 pH measurements with a Glass electrode • The glass electrode used to measure pH is the most common ion-selective electrode. • A typical pH combination electrode, incorporating both glass and reference electrodes in one body. • Glass combination electrode with a silver-silver chloride reference electrode. The glass electrode is immersed in a solution of unknown pH so that the porous plug on the lower right is below the surface of the liquid. The two silver electrodes measure the voltage across the glass membrane. • The potential difference between inner and outer silver-silver chloride electrodes depends on the chloride concentration in each electrode compartment and on the potential difference across the glass membrane. • Because [Cl−] is fixed in each compartment and because [H+] is fixed on the inside of the glass membrane, the only variable is the pH of analyte solution outside the glass membrane. • The voltage of the ideal pH electrode changes by 59.16 mV for every pH-unit change of analyte activity at 25°C. Errors in pH measurement 1. 2. 3. 4. 5. 6. 7. 8. 9. Standards. Junction potential Junction potential drift. Sodium error. Acid error. Equilibration time. Hydration of glass. Temperature. Cleaning. Ion-selective electrodes-example Most ion-selective electrodes fall into one of the following classes: 1. Glass membranes for H+ and certain monovalent cations 2. Solid-state electrodes based on inorganic salt crystals 3. Liquid-based electrodes using a hydrophobic polymer membrane saturated with a hydrophobic liquid ion exchanger 4. Compound electrodes with a species-selective electrode enclosed by a membrane that is able to separate that species from others or that generates the species in a chemical reaction. Ion-selective electrodes-example • Electrodes that respond selectively to specific analytes in solution or in the gas phase. • Ion exchange between heparin and Cl− associated with tetraalkylammonium ions in the membrane of the ion-selective electrode. Rotating ion-selective heparin electrode Potentiometry with an Oscillating Reaction • The Belousov-Zhabotinskii reaction is a cerium-catalyzed oxidation of malonic acid by bromate, in which the quotient [Ce3+]/[Ce4+] oscillates by a factor of 10 to 100. • • • • • Oscillation between yellow and colorless is set up in a 300-mL beaker with the following solutions: 160 mL of 1.5 M H2SO4 40 mL of 2 M malonic acid 30 mL of 0.5 M NaBrO3 (or saturated KBrO3) 4 mL of saturated ceric ammonium sulfate, (Ce(SO4)2 · 2(NH4)2SO4 · 2H2O) Trace b shows two different cycles superimposed in the same solution. This unusual event occurred in a reaction that had been oscillating normally for about 30 min