Slide 1 - Red Hook Central School District
advertisement

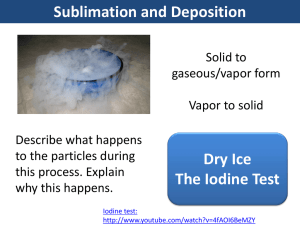
Colligative Properties Colligative Properties…. Are properties that depend on the number of dissolved particles only. The type of dissolved particles does not matter Freezing Point • The temperature at which a solution freezes from a liquid to a solid • Freezing point of pure water is 0o C • Adding a solute to pure water lowers the freezing point below zero Freezing Point is lowered when: • Salt is added to roads in winter, so they don’t get icy even if the temp is below zero • Antifreeze is added to engine fluids so they don’t freeze inside your engine and your car still starts when the temp is below zero Take 1000g of pure water And add one mole of dissolved particles, and you will lower the freezing point by 1.86 degrees C. Remember: it does not matter what the dissolved particles are, only how many there are!!! Boiling Point • The temperature at which a solution boils from a liquid to a gas • Boiling point of pure water is 100o C • Adding a solute to pure water raises the boiling point above 100 degrees C Boiling Point is raised when: • Salt is added boiling water to cook pasta faster (at a higher temperature) so the pasta does not get “soggy”. Instead it comes out “al dente” • Antifreeze is added to engine fluids so they don’t boil inside your engine and your car won’t overheat when temp is above 100 degrees C. Take 1000g of pure water And add one mole of dissolved particles, and you will raise the boiling point by 0.52 degrees C. Remember: it does not matter what the dissolved particles are, only how many there are!!! Some solutes are more effective than others at raising and lowering boiling and freezing points • I mole of sugar (covalent compound) dissolves in water to form 1 mole of dissolved sugar C6H1206(aq) • 1 mole of NaCl (ionic compound) will dissolve to form 2 moles of dissolved particles: 1 mole of Na+ (aq) and I mole of Cl- (aq) Some solutes are more effective than others at raising and lowering boiling and freezing points • 1 mole of CaCl2 (ionic compound) will dissolve to form 3 moles of dissolved particles: 1 mole of Ca+ (aq) and 2 moles of Cl- (aq) • In general, ionic compounds form more dissolved particles, so they are a better choice for adding to the water to change boiling or freezing points. Vapor Pressure: Table H some molecules at the surface of a liquid may have enough energy to evaporate even if the liquid is below its boiling point. These escaping gas particles exert a pressure called VAPOR PRESSURE. Vapor Pressure • The more easily the molecules evaporate, the higher the vapor pressure • The weaker the intermolecular forces, the more easily the molecules can separate and go into the gas phase, therefore the higher the vapor pressure Vapor Pressure • The more easily the molecules evaporate, the higher the vapor pressure • The stronger the intermolecular forces, the less easily the molecules can separate and go into the gas phase, therefore the lower the vapor pressure Using Table H • Which liquid has the highest vapor pressure at 50 degrees? • The lowest? • Which liquid has the weakest intermolecular forces? • The strongest? Vapor pressure The boiling point of a liquid is defines as the temperature at which the vapor pressure of the liquid equals atmospheric pressure (1 atm or 101.3 kPa) In other words, at that temp all the molecules change to gas phase (boil), not just the molecules at the surface