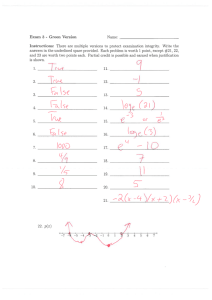
Writing Formulae

STANDARD GRADE CHEMISTRY
Writing Formulae.
Formulae of compounds can be written using either
1. The name of the compound.
“mono”
“di”
“tri”
“tetra”
“penta” means means means means means one two three four five
If any of these prefixes appears in the name of the compound we use this to write the formula.
e.g.
carbon monoxide is carbon dioxide is
CO
CO
2
2. Using Valency Rules
If the name does not give any clues about the formula, we use valency rules.
e.g.
sodium oxide write symbols write valency
Na
1
O
2 cross over valency cancel if necessary write formula
2
Na
2
O
1
Standard Grade Chemistry
Note:- For transition metals the valency is usually written as a Roman numeral in brackets after the name of the transition metal e.g. In iron(III) oxide, the iron has a valency of 3.
3. Formulae involving Complex (group) Ions
If the name of the compound ends in “-ate” or “ –ite” , it contains a complex ion (watch also for hydroxide). Complex ions are found in your data book (p4).
e.g. Copper(II) nitrate write symbols or formula write valency
(for complex ions, this is the charge)
Cu
2
NO
1
3
cross over valency
Use brackets and cancel if necessary write formula
1
Cu(NO
3
)
2
2
4. Formulae Showing Charges
Sometimes you are asked to show the formula with charges (ionic formula) e.g. sodium carbonate write symbols or formula write valency
(for complex ions, this is the charge) cross over valency
Use brackets and cancel if necessary write formula leaving charges
Na
1
2
(Na + )
2
CO
3
2-
CO
3
2-
2
1
Write ionic formulae for the compounds listed above.
Calculations for you to try.
1. Write the formula for:-
(a) Sulphur trioxide
(b) Dinitrogen monoxide
(c) Phosphorus pentachloride
(d) Carbon terachloride.
SO
3
N
2
O
PCl
5
CCl
4
Standard Grade Chemistry
2. Write the formula for:-
(a) Sodium oxide.
(b) Aluminium iodide
(c) Iron(III) oxide
(d) Silver(I) oxide.
Na
2
O
AlI
3
Fe
2
O
3
Ag
2
O
3. Write the formula for:-
(a) Sodium carbonate
(b) Aluminium nitrate
(c) Iron(III) hydroxide
(d) Nickel(II) sulphate
Na
2
CO
3
Al(NO
3
)
3
Fe(OH)
3
NiSO
4
4. Write the ionic formula for:-
(a) Lithium hydrogencarbonate
(b) Aluminium hydroxide
(c) Iron(II) sulphate
(d) Copper(II) nitrate.
Li + HCO
3
-
Al 3+ (OH )
3
Fe 2+ SO
4
2-
Cu 2+( NO
3
)
2