Organic Chemistry
advertisement
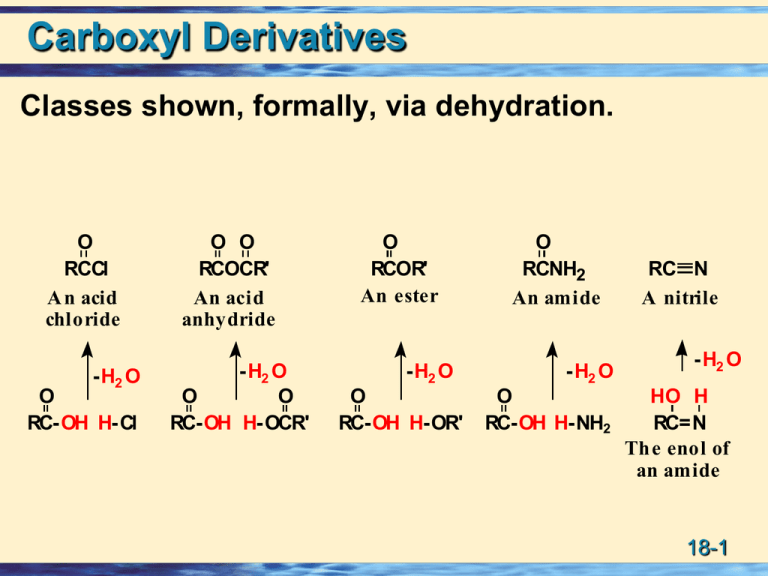
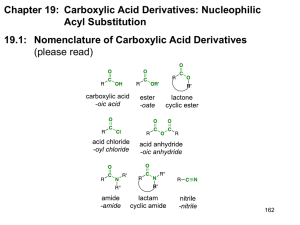
Carboxyl Derivatives Classes shown, formally, via dehydration. O RCCl A n acid chloride -H2 O O RC-OH H-Cl O O RCOCR' An acid anhydride -H2 O O O RC-OH H-OCR' O RCOR' An ester -H2 O O RC-OH H-OR' O RCNH2 An amide -H2 O O RC-OH H-NH2 RC N A nitrile -H2 O HO H RC=N Th e enol of an amide 18-1 Structure: Acid Chlorides The functional group of an acid halide is an acyl group bonded to a halogen. • The most common are the acid chlorides. • To name, change the suffix -oic acid to -oyl halide. O O RCAn acyl group O CH3 CCl O Cl Ethan oyl ch loride Benzoyl chloride (Acetyl ch loride) Cl Cl O Hexan edioyl ch loride (Adip oyl chloride) 18-2 Related: Sulfonyl Chlorides • Replacement of -OH in a sulfonic acid by -Cl gives a sulfonyl chloride. O CH3 SOH O Methanes ulfon ic acid O CH3 SCl O Methanes ulfonyl ch loride (Mesyl ch loride, MsCl) O H3 C SOH O p-Toluen esulfon ic acid O H3 C SCl O p-Toluen esulfon yl chloride (Tosyl chlorid e, TsCl) 18-3 Structure: Acid Anhydrides Two acyl groups bonded to an oxygen atom. • The anhydride may be symmetrical (two identical acyl groups) or mixed (two different acyl groups). • To name, replace acid of the parent acid by anhydride. O O O O CH3 COCCH3 COC Acetic anhydride Benzoic anhydride 18-4 Acid Anhydrides Cyclic anhydrides are named from the dicarboxylic acids from which they are derived. O O O S u cci n ic an h ydride O O O Male i c an h ydride O O O Ph th al ic an h ydride 18-5 Esters The functional group of an ester is an acyl group bonded to -OR or -OAr. • Name the alkyl or aryl group bonded to oxygen followed by the name of the acid. • Change the suffix -ic acid to -ate. O O O O O Ethyl ethan oate (Ethyl acetate) Is op ropyl ben zoate EtO OEt O D iethyl butaned ioate (D ieth yl s uccin ate) 18-7 Esters; Lactones Lactone: A cyclic ester. • name the parent carboxylic acid, drop the suffix -ic acid and add -olactone. 2 3 H3 C O O 1 O 3-Bu tanolactone -Butyrolactone) 1 2 O 3 4 4-Bu tanolactone -Bu tyrolacton e) 3 4 2 O 1 O 5 6 6-Hexan olacton e -Cap rolactone) 18-8 Amides The functional group of an amide is an acyl group bonded to a nitrogen atom. • drop -oic acid from the name of the parent acid and add -amide. (For the common acid name, drop -ic of the acid name and add -amide.) • an alkyl or aryl group bonded to the N: name the group and show its location on nitrogen by N-. O CH3 CNH2 A cetamide (a 1° amide) ethanamide O H CH3 C-N CH3 O CH3 H-C-N CH3 N-Methylacetamide N ,N-D imethyl(a 2° amid e) formamid e (DMF) (a 3° amide) 18-10 Amides: resonance 18-11 Amides; Characteristics 18-12 Amides; Lactams Lactams: A cyclic amides are called lactams. • Name the parent carboxylic acid, drop the suffix -ic acid and add -lactam. 2 3 H3 C O 1 NH 3-Bu tanol actam -Butyrol actam) 3 4 O 2 1 NH 5 6-He xan olactam -C aprolactam) 6 Indicates where the N is located. 18-13 Imides The functional group of an imide is two acyl groups bonded to nitrogen. • Both succinimide and phthalimide are cyclic imides. O NH O Succinimide O NH O Phthalimide 18-14 Related: Nitriles The functional group of a nitrile is a cyano group • IUPAC names: name as an alkanenitrile. • common names: drop the -ic acid and add -onitrile. CH3 C N Ethanen itrile (A cetonitrile) C N Benzon itrile CH2 C N Phenylethan enitrile (Phenylacetonitrile) 18-15 Acidity of N-H bonds Amides are comparable in acidity to alcohols. • Water-insoluble amides do not react with NaOH or other alkali metal hydroxides to form water-soluble salts. Sulfonamides and imides are more acidic than amides. O CH3 CNH2 Acetamide pKa 15-17 O SNH2 O NH O NH O O O Ben zenesu lfonamide Succinimide Phth alimide pK a 10 p Ka 9.7 p Ka 8.3 18-16 Acidity of N-H bonds Effect of neighboring carbonyl groups. 1.0 18-17 Acidity of N-H • Imides such as phthalimide readily dissolve in aqueous NaOH as water-soluble salts. O NH + O pK a 8.3 (stron ge r aci d) O N aOH - N Na + + H2 O O (stron ge r base ) (weak e r base ) pK a 15.7 (weak e r aci d) 18-18 Acidity of N-H bonds Imides are more acidic than amides because 1. the electron-withdrawing inductive of the two adjacent C=O groups weakens the N-H bond, and 2. More resonance delocalization of the negative charge. O N O O N O A resonance-stabilized an ion O N O 18-19 Lab related: Sulfonamides (Hinsberg) Experimental test to distinguish primary, secondary and tertiary amines. 1 soluble insoluble 2 insoluble 3 soluble In base In acid Reaction replaces one H with the sulfonyl group. In 18-20 an H remains it is soluble in base. Characteristic Reactions: Ketones & Aldehydes Nucleophilic acyl Addition: Protonation makes carbonyl better electrophile. Ok with poor nucleophile. Carbonyl weaker electrophile. Need good nucleophile. 18-21 Characteristic Reactions: Derivatives Nucleophilic acyl substitution: An additionelimination sequence resulting in substitution of one nucleophile for another. O O R C + Y :Nu R O C Nu R C + Nu :Y Y Te trah edral carbon yl S u bstitu tion addi tion i n te rme diate produ ct Dominant for derivatives due to good leaving group (Y), uncommon for ketones or aldehydes. 18-22 Characteristic Reactions Poor bases make good leaving groups. O R2 N- RO- RCO- X- Increasing leaving ability Increasing basicity Halide ion is the weakest base and the best leaving group; acid halides are the most reactive toward nucleophilic acyl substitution. Amide ion is the strongest base and the poorest leaving group; amides are the least reactive toward nucleophilic acyl substitution. 18-23 Water and Acid Chlorides • Low-molecular-weight acid chlorides react rapidly with water. • Higher molecular-weight acid chlorides are less soluble in water and react less readily. O CH3 CCl + H2 O Acetyl chlorid e O CH3 COH + HCl 18-24 Water and Anhydrides • Low-molecular-weight anhydrides react readily with water to give two molecules of carboxylic acid. • Higher-molecular-weight anhydrides also react with water, but less readily. O O CH3 COCCH3 + H2 O Acetic an hydrid e O O CH3 COH + HOCCH3 18-25 Mechanism- Anhydrides • Step 1: Addition of H2O to give a TCAI. (Addition) H + O CH3 -C- O-C- CH 3 O-H H H O O CH3 -C- O-C- CH 3 + O H H O-H H H O O + CH3 -C- O-C- CH 3 + H- O-H O H H Te trah edral carbon yl addi tion i n te rme diate Acid makes carbonyl better electrophile. 18-26 Mechanism- Anhydrides • Step 2: Protonation and collapse of the TCAI. (Elimination) H H +O H H H O H O O O CH3 -C-O-C-CH3 O H H H +O H + H O CH3 C O C CH3 H H H O CH3 O O C + O C O CH3 H Acid sets up better leaving group. 18-27 Water and Esters Esters are hydrolyzed only slowly, even in boiling water. • Hydrolysis becomes more rapid if they are heated with either aqueous acid or base. Hydrolysis in aqueous acid is the reverse of Fischer esterification. • acid catalyst protonates the carbonyl oxygen and increases its electrophilic character toward attack by water (a weak nucleophile) to form a tetrahedral carbonyl addition intermediate. • Collapse of this intermediate gives the carboxylic acid and alcohol. 18-28 Mechanism: Acid/H2O - Esters (1o and 2o alkoxy) Acid-catalyzed R O C ester hydrolysis. OH C + + OCH3 Acid makes carbonyl Better electrophile. H2 O H R H + OH R O C + OH CH3 OH OCH3 Tetrahed ral carbonyl ad dition intermed iate Acid sets up leaving group. 18-29 Mechanism: Reaction with Acid/H2O – Esters (3o alkoxy) But wait!!!!!!! water alcohol 18-30 Reaction with Base/H2O - Esters Saponification: The hydrolysis of an esters in aqueous base. • Each mole of ester hydrolyzed requires 1 mole of base • For this reason, ester hydrolysis in aqueous base is said to be base promoted. O RCOCH3 + NaOH H2 O O - RCO Na + + CH3 OH 18-31 Mechanism of Reaction with Base/H2O – Esters • Step 1: Attack of hydroxide ion (a nucleophile) on the carbonyl carbon (an electrophile). (Addition) • Step 2: Collapse of the TCAI. (Elimination) • Step 3: Proton transfer to the alkoxide ion; this step is irreversible and drives saponification to completion. O O O (1) (2) R- C + R- C-OCH3+ OH R- C OCH3 O OH H O OCH3 (3) R- C + HOCH3 O 18-32 Acidic Reaction with H2O - Amides Hydrolysis of an amide in aqueous acid requires one mole of acid per mole of amide. • Reaction is driven to completion by the acid-base reaction between the amine or ammonia and the acid. O O N H2 + H2 O + HCl Ph 2-Ph e n ylbu tan am ide H2 O heat OH + N H4 + Cl - Ph 2-Ph e n ylbu tan oi c aci d 18-33 Basic Reaction with H2O - Amides Hydrolysis of an amide in aqueous base requires one mole of base per mole of amide. • Reaction is driven to completion by the irreversible formation of the carboxylate salt. O CH3 CNH N-Phen yleth anamide (N-Phen ylacetamid e, Acetan ilide) + NaOH H2 O heat O CH3 CO- Na+ + H2 N Sodiu m acetate A niline 18-34 Mechanism: Acidic H2O - Amides • Step1: Protonation of the carbonyl oxygen gives a resonance-stabilized cation intermediate. O + R C NH2 + H O H H +H O R C NH2 H H O R C + O NH2 R C + NH2 + H2 O Reso nance-stabil ized catio n i ntermed iate 18-35 Acidic H2O - Amides • Step 2: Addition of water (a nucleophile) to the carbonyl carbon (an electrophile) followed by proton transfer gives a TCAI. OH + R C NH2 + OH O H H R C NH2 O+ H H proton transfe r from O to N OH R C NH3 + H O • Step 3: Collapse of the TCAI and proton transfer. (Elimination) H R C NH3 + OH H + O R O C + NH3 R O C + OH + NH4 OH 18-36 Mechanism: Reaction with Basic H2O - Amides Amide hydroxide ion Dianion! 18-37 Acidic H2O and Nitriles The cyano group is hydrolyzed in aqueous acid to a carboxyl group and ammonium ion. Ph CH2 C N + 2 H2 O + H2 SO4 Phenylacetonitrile H2 O heat O + Ph CH2 COH + NH4 HSO4 Ph enylacetic Ammoniu m acid hydrogen s ulfate • Protonation of the cyano nitrogen gives a cation that reacts with water to give an imidic acid. • Keto-enol tautomerism gives the amide. Acid OH O + + H + H O R-C N R-C NH R-C-NH 2 2 Ammonium A n imidic acid An amide ion (en ol of an amide) 18-38 Basic H2O and Nitriles • Hydrolysis of a cyano group in aqueous base gives a carboxylic anion and ammonia; acidification converts the carboxylic anion to the carboxylic acid. CH3 ( CH2 ) 9 C N Un decan enitrile NaOH, H2 O h eat O + CH3 ( CH2 ) 9 CO Na + NH3 S od ium und ecanoate HCl H2 O O CH3 ( CH2 ) 9 COH + NaCl + NH4 Cl Und ecanoic acid 18-39 Synthesis: Reaction with H2O - Nitriles • Hydrolysis of nitriles is a valuable route to carboxylic acids. CH 3 ( CH2 ) 8 CH2 Cl KCN ethanol, 1-C hlorode cane water CH3 ( CH2 ) 9 C N Unde cane nitril e OH H2 SO4 , H2 O heat O CH3 ( CH2 ) 9 COH Unde canoic aci d OH CHO HCN , KCN CN H2 SO 4 , H2 O COOH e th an ol, heat wate r Be n z al de hyde Be n z al de h yde cyan oh ydrin 2-H ydroxyph e n ylacetic aci d (Man de l on itri le ) (Man de l ic acid) (racemic) (racemic) 18-40 Synthesis: Grignards + Nitriles ->ketone 1 NMgX RC N R'MgX RCR' NH H2O RCR' diethyl ether Grignard reagents add to carbon-nitrogen triple bonds in the same way that they add to carbonoxygen double bonds. The product of the reaction is an imine. 18-41 Synthesis: Grignards + Nitriles ->ketone 2 NMgX RC N R'MgX RCR' NH H2O RCR' diethyl ether H3O+ Imines hydrolyzed to ketones. O RCR' 18-42 Reaction of Alcohols and Acid Halides Acid halides react with alcohols to give esters. • Acid halides are so reactive toward even weak nucleophiles such as alcohols that no catalyst is necessary. • Where the alcohol or resulting ester is sensitive to HCl, reaction is carried out in the presence of a 3° amine to neutralize the acid. O O Cl + HO Butanoyl chloride Cyclohexan ol O + HCl Cyclohexyl butan oate 18-43 Reaction with Alcohols, Sulfonic Esters • Sulfonic acid esters are prepared by the reaction of an alkane- or arenesulfonyl chloride with an alcohol or phenol. • The key point here is that OH- (a poor leaving group) is transformed into a sulfonic ester (a good leaving group) with retention of configuration at the chiral center. OT s OH + (R)-2-Octanol T sCl pyridine p-Toluenesulfonyl chloride (Tosyl chloride) (R)-2-Octyl p-t oluenesulfonate [(R)-2-Octyl tosylate] 18-44 Reaction of Alcohols and Acid Anhydrides Acid anhydrides react with alcohols to give one mole of ester and one mole of a carboxylic acid. O O CH3 COCCH3 + HOCH2 CH 3 Ace ti c an h ydride Eth an ol O O CH3 COCH2 CH3 + CH3 COH Ace ti c aci d Eth yl ace tate • Cyclic anhydrides react with alcohols to give one ester group and one carboxyl group. O O O O Phth alic anh yd rid e + O OH HO O 2-Butan ol (sec-Butyl alcohol) (s ec-Bu tyl h yd rogen phth alate 18-45 Reaction of Alcohols and Esters Esters react with alcohols in the presence of an acid catalyst in an equilibrium reaction called transesterification. O + OCH3 Me th yl prope n oate (Me th yl acrylate) (bp 81°C ) HO 1-Bu tan ol (bp 117°C ) HCl O O + CH3 OH Bu tyl prope n oate Me th an ol (Bu tyl acrylate) (bp 65°C ) (bp 147°C ) 18-46 Reaction of Ammonia, etc. and Acid Halides Acid halides react with ammonia, 1° amines, and 2° amines to form amides. • Two moles of the amine are required per mole of acid chloride. O O Cl + 2 NH3 Hexanoyl chloride Ammon ia + - NH2 + NH4 Cl Hexan amid e Ammon ium chloride 18-47 Reaction of Ammonia, etc. and Anhydrides. Acid anhydrides react with ammonia, and 1° and 2° amines to form amides. • Two moles of ammonia or amine are required. O O CH3 COCCH3 + 2 NH3 Acetic Ammon ia anh yd rid e O O + + CH CO NH CH3 CNH2 3 4 Acetamid e Ammon ium acetate 18-48 Ammonia, etc. and Esters Esters react with ammonia and with 1° and 2° amines to form amides. • Esters are less reactive than either acid halides or acid anhydrides. O Ph O OEt + NH3 Ethyl p henylacetate Ph NH2 + Phenylacetamide Et OH Ethanol Amides do not react with ammonia or with 1° or 2° amines. 18-49 Acid Chlorides with Salts Acid chlorides react with salts of carboxylic acids to give anhydrides. • Most commonly used are sodium or potassium salts. O O + CH3 CCl + N a - OC Ace tyl ch loride S odiu m be n zoate O O CH3 COC + N a+ Cl - Ace ti c be n z oic an h ydride 18-50 Interconversions of Acid Derivatives 18-51 Grignard and an Ester. Look for two kinds of reactions. O OMgX O O R-Mg-X R-Mg-X R' R' Any alcohol will do here. OEt OEt R' R R' R R R Substitution EtOH R'COCl But where does an ester come from? OH Acid chloride R' SOCl2 R'CO2H R R Perhaps this carboxylic acid comes from the oxidation of a primary alcohol or reaction of a Grignard with CO2. Addition 18-52 Grignard Reagents and Formic Esters • Treating a formic ester with two moles of Grignard reagent followed by hydrolysis in aqueous acid gives a 2° alcohol. O HCOCH3 + 2RMgX An ester of formic acid OH magnesium H O, HCl 2 alkoxide HC-R + CH3 OH salt R A 2° alcohol 18-53 Reactions with RLi Organolithium compounds are even more powerful nucleophiles than Grignard reagents. O RCOCH3 1 . 2 R' Li 2 . H2 O, HCl OH R- C-R' + CH3 OH R' 18-54 Gilman Reagents Acid chlorides at -78°C react with Gilman reagents to give ketones. O O 1 . ( CH3 ) 2 CuLi, eth er, -78°C Cl 2 . H O 2 Pe n tan oyl ch l ori de 2-H exan on e Gilman Reagents do not react with acid anhydrides, esters, amides or nitriles under these conditions. Selective reaction. 18-55 Synthesis: Reduction - Esters by LiAlH4 Most reductions of carbonyl compounds use hydride reducing agents. • Esters are reduced by LiAlH4 to two alcohols. • The alcohol derived from the carbonyl group is primary. O Ph OCH3 Me th yl 2-ph en yl propan oate (race mic) 1 . LiA lH4 , e t he r 2 . H2 O, HCl Ph OH + CH3 OH 2-Ph e n yl-1propan ol (race mic) Me th an ol 18-56 Mechanism: Reduction - Esters by LiAlH4 Reduction occurs in three steps plus workup: • Steps 1 and 2 reduce the ester to an aldehyde. O R C OR' + H (1) O R C OR' (2) O R C H + OR' H A tetrahedral carbonyl addition intermediate • Step 3: Work-up gives a 1° alcohol derived from the carbonyl group. O R C H + H (3) O R C H H OH (4) R C H H A 1° alcohol 18-57 Synthesis: Selective Reduction by NaBH4 NaBH4 reduces aldehydes and ketones. It does not normally reduce esters. LiAlH4 reduces all. Selective reduction is often possible by the proper choice of reducing agents and experimental conditions. O O OEt NaBH4 OH O EtOH OEt (racemic) 18-58 Synthesis: Reduction - Esters by DIBAlH -> Aldehyde Diisobutylaluminum hydride (DIBAlH) at -78°C selectively reduces an ester to an aldehyde. • At -78°C, the TCAI does not collapse and it is not until hydrolysis in aqueous acid that the carbonyl group of the aldehyde is liberated. O 1 . DIBALH , toluen e, -78°C OCH3 2 . H2 O, HCl Me th yl h e xan oate O H + CH3 OH He xan al 18-59 Synthesis: Reduction - Amides by LiAlH4 LiAlH4 reduction of an amide gives a 1°, 2°, or 3° amine, depending on the degree of substitution of the amide. O 1 . LiAlH4 NH2 2 . H O 2 Octanamide NH2 1-Octanamine O NMe2 1 . LiAlH4 2 . H2 O N,N -D imethylben zamide NMe2 N ,N-D imeth ylb enzylamine 18-60 Synthesis: Reduction - Nitriles by LiAlH4 The cyano group of a nitrile is reduced by LiAlH4 to a 1° amine. 1 . LiA lH4 CH3 CH= CH( CH 2 ) 4 C N 2 . H2 O 6-O cte n en i tri le CH3 CH= CH ( CH2 ) 4 CH2 N H2 6-O cte n -1-am in e Can use catalytic hydrogenation also. 18-61 Interconversions Problem: Show reagents and experimental conditions to bring about each reaction. O Ph Cl (a) (b ) O Ph OH Ph enylacetic acid O O (d ) Ph (c) (e) Ph OMe (g) (f) Ph OH NH2 (h ) Ph NH2 18-62