Here is the Original File
advertisement
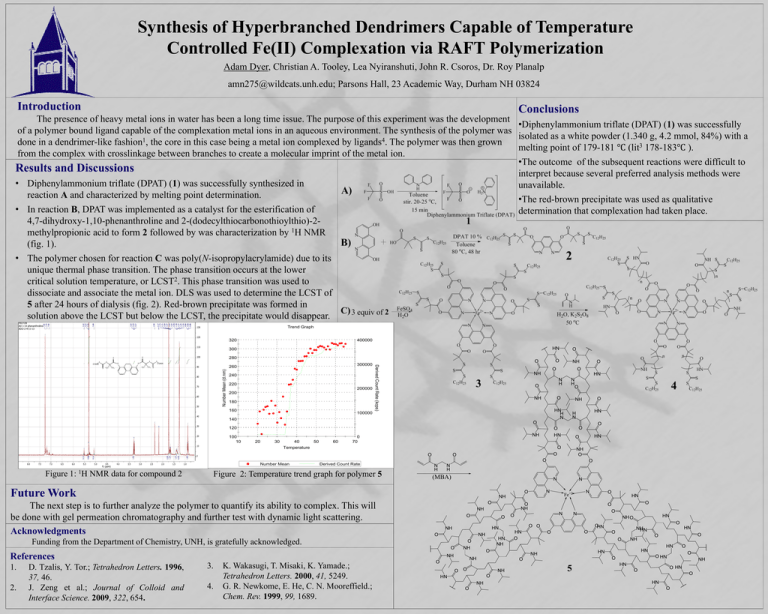
Synthesis of Hyperbranched Dendrimers Capable of Temperature Controlled Fe(II) Complexation via RAFT Polymerization Adam Dyer, Christian A. Tooley, Lea Nyiranshuti, John R. Csoros, Dr. Roy Planalp amn275@wildcats.unh.edu; Parsons Hall, 23 Academic Way, Durham NH 03824 Introduction Conclusions The presence of heavy metal ions in water has been a long time issue. The purpose of this experiment was the development •Diphenylammonium triflate (DPAT) (1) was successfully of a polymer bound ligand capable of the complexation metal ions in an aqueous environment. The synthesis of the polymer was isolated as a white powder (1.340 g, 4.2 mmol, 84%) with a 1 4 done in a dendrimer-like fashion , the core in this case being a metal ion complexed by ligands . The polymer was then grown melting point of 179-181 ℃ (lit3 178-183℃ ). from the complex with crosslinkage between branches to createTrend a molecular Temperature Reportimprint of the metal ion. v2.0 •The outcome of the subsequent reactions were difficult to Results and Discussions interpret because several preferred analysis methods were • Diphenylammonium triflate (DPAT) (1) was successfully synthesized in unavailable. reaction A and characterized by melting point determination. •The red-brown precipitate was used as qualitative Sample Details • In reaction B, DPAT was implemented as a catalyst forSample the esterification of Name: diluted Hot 4-25-13 determination that complexation had taken place. SOP Name: mansettings.nano 4,7-dihydroxy-1,10-phenanthroline and 2-(dodecylthiocarbonothioylthio)-2General Notes: 11.25 g/L methylpropionic acid to form 2 followed by was characterization by 1H NMR (fig. 1). File Name: Undiluted Hot 4-24-13.dts Dispersant Name: Water Record Number: 50 Dispersant RI: 1.330 • The polymer chosen for reaction C was poly(N-isopropylacrylamide) due toMeasurement its Material RI: 1.59 Date and Time: Thursday, April 25, 2013 2:1... Material Absorbtion: 0.01 unique thermal phase transition. The phase transition occurs at the lower critical solution temperature, or LCST2. This phase System transition was used to Temperature Start (°C): 20.0 Cell Description: Disposable sizing cuvette dissociate and associate the metal ion. DLS was used to determine the LCST of Temperature End (°C): 65.0 5 after 24 hours of dialysis (fig. 2). Red-brown precipitate was formed in Results solution above the LCST but below the LCST, the precipitate would disappear. Malvern Instruments Ltd - © Copyright 2008 Trend Graph 320 400000 300 280 Number Mean (d.nm) 240 220 200000 200 180 160 Derived Count Rate (kcps) 300000 260 100000 140 120 100 10 20 30 40 50 60 0 70 Temperature Number Mean Figure 1: 1H NMR data for compound 2 Derived Count Rate Figure 2: Temperature trend graph for polymer 5 Future Work The next step is to further analyze the polymer to quantify its ability to complex. This will be done with gel permeation chromatography and further test with dynamic light scattering. Acknowledgments Malvern Instruments Ltd www.malvern.com Zetasizer Ver. 6.12 Serial Number : MAL500553 Funding from the Department of Chemistry, UNH, is gratefully acknowledged. References 1. 2. D. Tzalis, Y. Tor.; Tetrahedron Letters. 1996, 37, 46. J. Zeng et al.; Journal of Colloid and Interface Science. 2009, 322, 654. 3. 4. K. Wakasugi, T. Misaki, K. Yamade.; Tetrahedron Letters. 2000, 41, 5249. G. R. Newkome, E. He, C. N. Mooreffield.; Chem. Rev. 1999, 99, 1689. # Temp °C 50 51 20.0 52 21.0 53 22.0 54 23.0 55 24.0 56 25.0 57 25.9 58 27.0 59 28.0 60 29.0 61 30.1 62 31.1 63 31.9 64 33.0 65 34.0 66 35.0 67 36.0 68 37.0 69 38.0 70 39.0 71 40.0 72 41.0 73 42.0 74 43.0 75 44.0 76 45.0 77 46.0 78 47.0 79 48.0 80 49.0 81 50.0 DCR kcps 8685.2 8521.2 8676.5 9082.2 8565.4 9417.2 8619.9 9098.3 8835.7 9328.7 8829.0 9074.8 8498.2 8569.3 8842.6 103233.1 86901.1 209025.7 206955.1 222532.6 241846.6 258450.5 260526.4 288589.4 302523.4 319079.5 328949.6 334158.7 334280.9 331853.7 334087.6 Z-Avg d.nm 487.7 461.8 531.4 554.6 474.9 544.7 431.5 515.7 469.2 521.1 463.0 504.9 438.2 387.0 396.1 212.1 249.9 269.7 283.5 296.0 310.5 318.5 322.1 324.9 335.9 340.3 343.1 350.5 345.9 File name: Undiluted Hot 4-24-13 349.1 Record Number: 50 30 Apr 2013 1:16:10 PM 352.2











